Purpose
Brain metastases are the most common malignant tumors of the central nervous system. Up to 20-40% of patients with cancer develop brain metastases at some point during the course of disease, and these patients generally have a very poor prognosis [1, 2]. At present, treatment methods of brain metastases mainly include chemo-radiotherapy, radio-surgery, and surgery. Radio-surgery is an effective treatment option for brain metastases; however, it is not suitable for brain metastases larger than 4 cm in diameter [3, 4]. Due to the existence of blood-brain barrier and the limitation of normal brain tissue tolerance for radiotherapy, therapeutic efficacy in some brain metastases is not satisfactory, especially for larger metastatic tumors [5]. A recurrence occurs in a large proportion of patients. Recurrent brain metastases are often resistant to chemotherapy, resulting in reduced tumor response with a median survival of only 3.5-6.6 months [6]. Re-irradiation for recurrent metastases that have been treated with radiotherapy has a potentially high-risk of neuro-cognitive deterioration and radio-necrosis [7-9]. Because of the presence of primary tumors or underlying diseases, most patients with recurrent brain metastases have poor performance status and cannot tolerate surgical resection. Metastases located in eloquent areas of the brain cannot be treated by surgical resection. Therefore, surgical treatment is only suitable for a small proportion of patients with brain metastases [10, 11]. The exploration of treatment methods for recurrent brain metastases would be of great clinical value.
Iodine-125 (125I) brachytherapy is a loco-regional treatment technique applied to brain tumors, such as circumscribed gliomas and brain metastases [12-15]. Some studies have shown that 125I brachytherapy is a minimally invasive and effective treatment option that can improve survival, and is less restricted by tumor localization or size [11, 16, 17]. Although the effectiveness of this technique has been demonstrated, this approach has significant shortcomings. Firstly, the guidance of 125I seed implantation for brain metastases is mainly by stereotactic frame. Despite the assistance of a stereotaxic frame, deviations in the spatial distribution of 125I seeds sometimes occur [18, 19]. Secondly, the highly technical nature and complexity of this technique significantly limit its’ clinical application in brain metastases. In a previous study from our group, we invented a simplified surgical procedure using a novel three-dimensional template for 125I seed implantation in glioma, which demonstrated an improved overall survival in patients with recurrent glioblastoma [20]. As gliomas and brain metastases have different radiobiological characteristics and different target delineations in treatment planning, clinical outcomes and complications of gliomas and brain metastases are different. It is worth investigating whether patients with recurrent brain metastases can also achieve good clinical outcomes using this new 125I seed implantation method. In the current study, we combined a novel individualized three-dimensional template with 1.0-T open MR imaging to guide 125I seed implantation for recurrent brain metastases. The feasibility, safety, and efficacy of this technique were investigated, aiming to explore a new treatment method for patients with brain metastases.
Material and methods
Patients
Twenty-eight patients with recurrent brain metastases underwent 125I seed implantation guided by a three-dimensional template and open MR from December, 2017 to January, 2021. Thirty-eight brain metastases were treated. The median interval from initial treatment to intra-cranial recurrence was 9 months (range, 4-34 months). The median age of patients was 59 years (range, 36-76 years). The median tumor diameter was 3.4 cm (range, 1.9-6.0 cm). The basic characteristics of all the 28 cases with 38 metastases are shown in Table 1.
Table 1
Characteristics of the 28 patients with 38 metastases
Eligibility criteria were: 1) Patients with well-controlled primary tumors; 2) Recurrent brain metastases confirmed by pathology or magnetic resonance imaging (MRI); 3) The number of brain metastases ≤ 3; 4) Patients with an estimated survival time of > 3 months. Exclusion criteria were: 1) Karnofsky performance score < 40; 2) Platelet count < 50 × 109/l or severe coagulation dysfunction (international standard ratio > 1.5 or an activated partial thromboplastin time of twice more than the normal time); 3) Unconscious patients; 4) Patients with MRI contraindications. This study was approved by the institutional review board, and written informed consent was obtained from each patient.
Equipment and technique
Iodine-125 radioactive seeds (Xinke Pharmaceutical Co., Ltd., Shanghai, China) with an initial dose-rate of 7.7 cGy/h were used for brachytherapy. Seed implanting brachytherapy system (BTPS) (Beijing University of Aeronautics and Astronautics, Beijing, China) was applied for pre-operative treatment planning. A 1.0-T open MR (Philips Healthcare, Amsterdam, The Netherlands) was utilized to guide the brachytherapy procedure. A MR-compatible electrocardiogram monitor (Mammendorfer Institute of Physics and Medicine GmbH, Munich, Germany) was equipped to monitor vital signs of the patients. High-speed twist drills with a diameter of 1.9 mm were used to penetrate the skull bone. MR-compatible 18-gauge ball-tipped needles were applied for puncturing and implantation of 125I seeds. Individualized three-dimensional non-coplanar template (Zhuoye Electronic Technology Co., Ltd., Zibo, China) with an accuracy of 0.1 mm was used to assist the brachytherapy procedure.
Pre-operative preparation
Fish oil capsule markers were placed on the scalp as the alignment reference points for the three-dimensional template before pre-operative MR scanning. For cystic brain metastases, intra-tumoral cyst fluid was aspirated by the guidance of MR fluoroscopy before pre-operative MR scanning (Figure 1). Brachytherapy treatment plan was generated according to pre-operative contrast-enhanced T1-weighted isovoxel images, with a slice thickness of 1 mm. Gross tumor volume (GTV) was delineated according to an enhanced margin of tumor. Clinical target volume (CTV) contour was delineated 5 mm outside of GTV. Prescribed dose was 120-165 Gy. Iodine-125 seeds in a single form with low activity of 0.8 mCi were used. Spacing of the 125I seeds and needle paths were determined according to target volume of the tumor. Dosimetry parameters (D90, V100, and CI) were obtained to evaluate dose distributions. Conformity index (CI) was calculated for evaluating the conformity of dose distributions.
Fig. 1
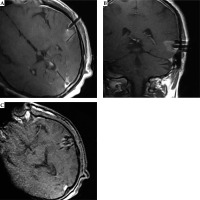
A 56-year-old man with a recurrent cystic metastasis in the left temporal lobe underwent real-time MRI-guided aspiration. A, B) The position of the needle was displayed by transverse and coronal T1W MR images. C) MRI showing shrinkage of the tumor after aspiration
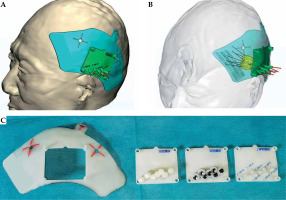
Based on brachytherapy treatment plan and contrast-enhanced T1-weighted images with slice thickness of 1 mm, an individualized three-dimensional non-coplanar template was printed by a 3D printer. The three-dimensional template was comprised of a base plate, a positioning template, a drilling template, and a puncturing template. The templates were used for fixation, position confirmation, cranial drilling, and puncturing, respectively, during the brachytherapy procedure (Figure 2).
Fig. 2
Pre-operative design and structure of 3D template. A, B) Pre-operative design of 3D template. C) The structure of 3D template showing the base plate, positioning plate, drilling plate, and puncturing plate (from left to right). The positioning plate, drilling plate, and puncturing plate are of the same size, and can be embedded in and removed from the base plate
Brachytherapy procedure
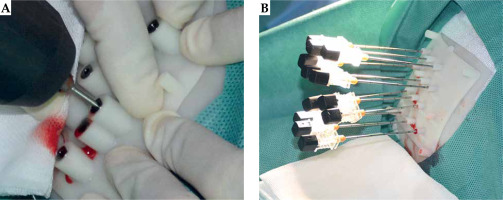
Iodine-125 seeds were implanted into brain metastases under the guidance of a 1.0-T open MR and three-dimensional template. The template was placed on the scalp coinciding with reference points. The position of the template was confirmed by enhanced MR images of T1-weighted turbo spin echo (T1W-TSE). Patient was then moved outside of the magnet bore, and lidocaine for local anesthesia and diazepam for intravenous conscious sedation were administered. Twist-drill holes were made in the skull through the drilling template using a battery-operated twist drill (Figure 3A). Then, needles with blunt tip were inserted into the tumor to pre-determined depths through the template (Figure 3B). The patient was moved back into the magnet bore and underwent MR scanning to visualize the position of needles (Figure 4). Iodine-125 seeds were implanted into the tumor following the pre-operative brachytherapy treatment plan.
Post-operative dosimetry verification
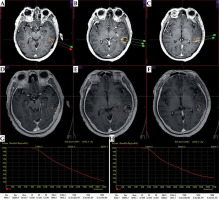
Post-operative dosimetry was verified using the brachytherapy treatment planning system based on fusion images of post-operative CT and MR (Figure 5). Dosimetry parameters of D90, V100, and CI were then obtained.
Fig. 5
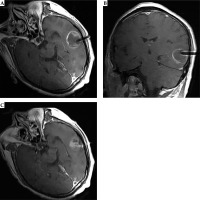
Pre-operative plan and post-operative dosimetry verification. A-C) Pre-operative treatment plan. D-F) Post-operative dosimetry verification based on post-operative CT/MR fusion images. G) Dose volume histogram of pre-operative plan. H) Post-operative dose volume histogram. Post-operative dosimetry matched the pre-operative treatment plan
Clinical follow-up
Patients were followed up every 2 to 3 months and underwent enhanced MRI. Clinical efficacy was evaluated as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) according to response assessment in neuro-oncology (RANO). Toxicities were recorded and evaluated according to Radiation Therapy Oncology Group (RTOG)/European Organization for Research and Treatment of Cancer (EORTC), and Common Terminology Criteria for Adverse Events (CTCAE) v. 4.0.
Statistical analysis
Pre- and post-operative D90, V100, and CI were compared using a paired t-test or non-parametric Wilcoxon test. Overall response rate (ORR), disease control rate (DCR) at 6 months, and 1-year survival rate were calculated. ORR was calculated as CR + PR/all evaluated patients. DCR was calculated as CR + PR + SD/all evaluated patients. Median overall survival (OS) measured from the date of 125I seed brachytherapy was estimated by Kaplan-Meier method. Statistical analysis was performed by SPSS v. 22.0 statistical software. P-value < 0.05 was considered statistically significant.
Results
Dosimetry verification
The pre- and post-operative D90 were 153.2 ±17.2 Gy and 154.7 ±15.1 Gy, respectively. The pre- and post-operative V100 were 92.1 ±1.5% and 92.2 ±2.3%, respectively, and the pre- and post-operative CI were 0.73 ±0.07 and 0.72 ±0.06, respectively. There were no statistically significant differences between pre- and post-operative dosimetry parameters of D90, V100, and CI, with p-values of 0.298, 0.804, and 0.053, respectively (Table 2).
Table 2
Comparison of pre-operative and post-operative dosimetry parameters
Local control and survival
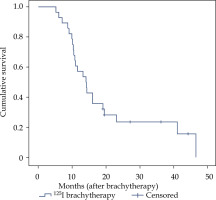
The follow-up time for all the patients was 5.2-46.5 months, and the median follow-up time was 14.2 months. Five patients (17.9%) were alive at the end of follow-up. Twelve patients (42.9%) died of systemic progression of primary tumors or extra-cerebral metastases. Nine patients (32.1%) died of cerebral progression, including 1 patient who died due to a progression of tumor treated with 125I brachytherapy. Six patients died due to new brain metastases in other cerebral areas, and 2 patients died of meningeal metastases. For two patients, further information about the cause of death were not available. Twenty-three patients had MR images for evaluation of clinical efficacy at 6 months. There were 11 cases of CR, 10 cases of PR, 1 case of SD, and 1 case of PD at 6 months (Figure 6). The ORR at 6 months was 91.3% (21/23) and the DCR at 6 months was 95.7% (22/23). The 1-year survival rate was 57.1% and the median OS was 14.1 months (95% confidence interval: 10.6-17.6) (Figure 7).
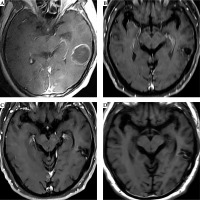
Fig. 6
MRI follow-up of the case shown in Figure 1. A) An enhanced T1W image before 125I brachytherapy treatment. B) T1W image six months after 125I brachytherapy showed that the tumor shrank, and the enhancement of the tumor decreased significantly. C, D) Enhanced and plain T1W images 30 months after brachytherapy showed no obvious enhancement of the tumor indicating a complete response
Complication and adverse reactions
There were two cases of small amount of hemorrhage, including 1 case of subdural hemorrhage and 1 case of intra-parenchymal hemorrhage. The bleeding volumes were about 1.8 ml and 2.1 ml, respectively. The two cases of hemorrhage were treated with mannitol and hemostatic drugs for 4 and 5 days. The complication rate was 7.1% (2/28). There were 5 cases of symptomatic brain edema after 125I seed implantation, including 3 cases of grade 3 edema and 2 cases of grade 2 edema. The patients’ symptoms were significantly relieved after 7 to 14 days of glucocorticoid and mannitol treatment. No patients suffered from a space-occupying radiation-induced necrosis requiring micro-surgical intervention. One case of epilepsy 2 months after brachytherapy was well-controlled with anti-epileptic medication.
Discussion
Iodine-125 seed implantation is a minimally invasive and effective treatment for brain tumors in addition to surgery and radiotherapy. Many clinical reports showed good clinical efficacy for 125I brachytherapy in brain metastases [11, 16, 17, 21, 22]. Ruge et al. reported that the overall response rate after 125I seed implantation for brain metastases was 94.6%, and was not significantly different between radio-sensitive and radio-resistant metastases [11]. Although 125I seed implantation for brain tumors has been used in clinical practice for many years, it has not been widely used in clinical practice due to the highly technical nature of implantation procedure.
Currently, for brain tumors, this treatment technique is restricted to only a few experienced medical institutions. Previous implantation procedures were mainly guided by stereotactic frame. There were non-negligible limitations to this technique. It was not possible to observe whether the position of puncture needles was accurate in real-time during brachytherapy procedure as the absence of real-time image feedback, resulting in deviations of 125I seed distributions [18, 19]. Additionally, for large-volume tumors, stereotactic 125I implantation requires adjusting coordinates of the frame repeatedly, and it is difficult to puncture using multi-needle. To solve these limitations, we invented a novel three-dimensional template that was used to guide 125I seed implantation in the treatment of brain tumors. This approach demonstrates good therapeutic effect in the treatment of recurrent glioblastoma [20]. Also, we investigated 125I seed implantation guided by the novel three-dimensional template and open MR for the treatment of recurrent brain metastases.
In this study, no statistically significant differences were observed between pre-operative and post-operative dosimetry parameters (D90, V100, and CI), indicating that 125I seeds were accurately distributed in the tumor through the assistance of three-dimensional template and the guidance of magnetic resonance. Therefore, the pre-operative brachytherapy treatment plan and accurate dosimetry distribution could be achieved. With the aid of the template, the puncture needles could be accurately inserted into the target of the tumor. Moreover, intra-operative MR imaging could detect a possible small deviation of the puncture needle that could be corrected in time to further ensure the accuracy of position of the puncture needle.
The ORR and DCR at 6 months were 91.3% and 95.7%, respectively, in this study, which showed good local control for recurrent brain metastases. We analyzed that there were at least two reasons. Firstly, the median prescribed dose applied in this study was 150 Gy, which was significantly higher than a dose given in previous studies. In most of the previous reports, the prescribed doses were ranging between 50 Gy and 132 Gy [11, 16, 23-25]. That was beneficial for local control of the tumors. Secondly, due to the assistance of template and guidance of intra-operative MRI, dose distribution is more conformal, dosimetric cold spots are largely avoided, and the tumor can be better covered by the prescribed dose. The median OS in this study was 14.1 months. Overall, our approach demonstrated good performance for 125I brachytherapy for recurrent brain metastases, and was comparable to previous studies that have reported OS ranging from 6 and 14.8 months [11, 16, 17, 24, 26, 27].
In the current study, brain edema was reported in 5 cases, in which the clinical symptoms were significantly alleviated after glucocorticoid and mannitol treatment. Of these 5 cases, 4 patients received SRS, and 1 patient received WBRT indicating that the patients who had received radiotherapy before 125I brachytherapy were more likely to develop brain edema. However, the clinical follow-up showed that this process was temporary and often occurred within 1 month after 125I seed implantation. There was no refractory brain edema or radio-necrosis induced by 125I brachytherapy, which is better compared with previous reports [17, 22]. Raleigh et al. performed 125I brachytherapy in 44 recurrent brain metastases after maximal tumor resection, and reported a 25% incidence of brain radio-necrosis (11/44) [17]. The low-risk of toxicities in our study may be due to several reasons. Firstly, with the assistance of individualized three-dimensional templates, 125I seeds could be accurately implanted into the tumor to achieve pre-operative treatment plan. Dose distributed in normal brain tissue and damage to normal brain tissue could be reduced. Secondly, the 125I seeds used were low-activity, which enabled dose distribution to be more conformal and not easy to form dose hot spots. When low activity seeds are used and tumor volume is large, more needle paths and twist-drill holes are necessary. Twist-drill holes can be accomplished at one time simply and rapidly under the guidance of a template to ensure the whole brachytherapy procedure is more straight forward. In this study, the incidence of cerebral hemorrhage was low. Analysis of the enhanced isovoxel MR images for the pre-operative brachytherapy treatment plan and the design of three-dimensional template avoided blood vessels located on the needle path to reduce cerebral hemorrhage.
At present, stereotactic 125I seed implantation requires the installation of head frame and stereotaxic instrument. An intra-operative procedure is complex, particularly for large tumors, and when more needle paths and twist-drill holes are needed. For these reasons, the application of stereotactic 125I seed implantation for brain tumors is limited to only a few experienced neurosurgeons, and the technique is not widely used in clinical practice. The three-dimensional template-assisted implantation technique applied in this study makes the brachytherapy procedure more programmatic, and significantly reduces technical difficulties. It is relatively easier to perform and is more beneficial for clinical application of brachytherapy for brain metastases. Therefore, patients with brain metastases who are suitable for 125I seed implantation may have more opportunities to receive their treatment. Although several steps are required to design and fabricate a three-dimensional template before brachytherapy; the process is programmable and not complicated.
Brachytherapy for brain tumors with seed implantation continues to be increasingly explored in clinical practice. Studies have shown that the application of a relatively novel radioisotope of cesium-131 (131Cs) can reduce the occurrence of brain necrosis [28, 29]. Yondorf et al. reported a prospective phase I/II trial demonstrating the intra-operative application of 131Cs brachytherapy for patients with newly diagnosed brain metastases. The study found that 131Cs is dosimetrically superior to 125I, with higher homogeneity indices (HI). The incidence of radiation necrosis was 0%, and a high-rate of local control (100%) was achieved [28]. Therefore, the application of a three-dimensional template combined with 131C seed in the treatment of brain metastases is worth investigating.
This study has several limitations. This was not a randomized controlled study, and a relatively small number of patients were enrolled. Further validation of our findings is required through randomized controlled studies with larger cohort of patients.
Conclusions
Three-dimensional template combined with MR-guided 125I seed implantation in the treatment of recurrent brain metastases is feasible, safe, and effective. The described method is relatively easy to perform and beneficial for clinical application. This novel 125I brachytherapy can be an attractive alternative in the treatment of brain metastases.