Purpose
Obstructive jaundice can arise from both malignant and benign sources, with the primary causes of malignant bile duct obstruction (MBO) being cholangiocarcinoma, gall bladder carcinoma, pancreatic adenocarcinoma, metastases of other tumors, or common bile duct (CBD) compression by proximal lymph nodes [1]. If not treated in a timely and effective manner, the obstruction can quickly lead to deterioration of liver, renal function, and blood coagulation, endangering patient’s life [2]. Resection is the most efficacious treatment option; however, many patients are not right candidates for this surgical procedure owing to their advanced disease state [3,4]. Adequate biliary drainage was the first selective palliative treatment method developed for patients with MBO, and some guidelines recommend the percutaneous approach over endoscopic retrograde cholangiography (ERC) for Bismuth type 3 and 4 hilar strictures [5]. However, this treatment has no therapeutic effect on tumors [6,7]. In recent years, intracavitary brachytherapy has been widely applied clinically, especially in patients with MBO [8]. This study investigated whether percutaneous transhepatic biliary drainage (PTBD) combined with iodine-125 (125I) stranded seeds can achieve good clinical effect.
Material and methods
Patients
The study protocol was approved by an institutional ethical committee (LDYYLL2018-115) and an informed consent was obtained from every patient before enrollment. A retrospective analysis was performed using the institutional database from May 2014 to May 2016 and was accessed to identify patients with MBO who underwent PTBD combined with 125I stranded seeds or PTBD. Patients’ inclusion criteria involved clinically or histopathologically confirmed malignant obstructive jaundice and with symptoms of jaundice, pruritus, cholangitis, or pain. Computed tomography (CT), magnetic resonance imaging (MRI), or ultrasound were conducted in all patients prior to the interventional procedure. Patients were of normal consciousness, cooperative, with the Eastern Cooperative Oncology Group (ECOG) performance status score of 0-3. Exclusion criteria included biliary tract strictures that were uncrossable, perforation of ducts within the biliary tree, ECOG performance of 4, contrast medium allergies, renal dysfunction, previous surgical treatment, or were non-cooperative with treatment. Chemotherapy was not an exclusion criterion for enrollment.
Finally, the study population comprised of 58 patients, in which PTBD was performed with an internal-external drainage tube. After all patients underwent initial PTBD, the 125I stranded seeds treatment strategy was recommended by the attending physician. If the patient agreed to the recommendation, 125I stranded seeds were administered. Patients who refused 125I stranded seeds were treated with PTBD alone. Finally, 20 of the 58 patients who underwent PTBD combined with 125I stranded seeds were included in group A. A total of 38 patients were comprised in group B, who were treated with PTBD alone.
Internal-external drainage tubes from Create Medic Co. (Kanagawa, Japan) were used. Iodine-125 seeds (4.5 mm × 0.8 mm, 1.70 cm effective functional diameter; 0.58 mci activity with a half-life of 59.6 days and 27.4-35.5 keV of radiation energy) from Shanghai GMS Pharmaceutical Co., Ltd. (China) were also used.
Procedure
All procedures were performed by a highly experienced surgeon with an expert knowledge of both PTBD and PTBD combined with 125I stranded seeds procedures. CT, MRI, or ultrasound confirmed the planned puncture pathway. An epigastric approach was employed for punctures of the left biliary system, whilst a right-sided intercostal approach was employed for punctures of the right biliary system to guarantee the stability of drainage catheter and to reduce the risk of bleeding. First, local anesthetic was injected (5-10 ml of xylocaine), using a 22-gauge spinal needle positioned subcutaneously and up to the liver capsule. The intrahepatic biliary duct (right or left) was punctured with a 22-gauge Chiba needle using a micropuncture kit (Cook Medical Inc., Bloomington, USA) under ultrasound guidance. A micro guidewire was passed and advanced as close as possible to the stenosis/occlusion under fluoroscopic. The Chiba needle was exchanged for a 4-Fr coaxial dilator, and a working cannula was positioned. A guidewire and a 5-Fr cobra catheter (Terumo, Tokyo, Japan) crossed the stenosis/occlusion with different tip morphologies, according to anatomical findings. Finally, a unilateral (right or left duct involvement or low bile duct obstruction) or bilateral (hilar hepatic duct lesions involving both left and right hepatic ducts) drainage tubes (Cook Medical Inc., Bloomington, USA) were placed across the stenosis/occlusion, with the distal tip reaching the duodenum.
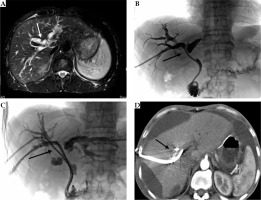
Seven days post-implantation of the drainage tube, group A underwent implantation of 125I stranded seeds. To manufacture the 125I stranded seeds, a flexible stiffening cannula (Create Medic Co., Kanagawa, Japan) with a sealed tip was utilized. The 125I seeds were arranged in the cannula to prepare the seed strand. The 125I stranded seeds were then sent through an internal-external drainage tube under fluoroscopic surveillance to allow appropriate placement at the site of obstruction. None of these procedures were performed under anesthesia. The length of 125I stranded seeds was designed to be slightly longer than the obstruction to ensure full exposure (Figure 1). Finally, the internal-external drainage tube and the end of the 125I stranded seeds were fixed onto the skin surface. Protective radiological procedures were performed according to the criteria recommended by the International Commission on Radiological Protection (ICRP) [9]. Exposure was minimized and protective vests were worn.
Fig. 1
A 51-year-old male with cholangiocarcinoma, Bismuth-Corlette type III. Preoperative MRI (A) showed intrahepatic bile duct dilation. Percutaneous transhepatic biliary drainage (PTBD) was performed with one internal-external drainage tube under fluoroscopic monitoring. Imaging showed (B) substantial narrowing at the hepatic duct. One week later, two of the 125I stranded seeds were placed through the internal-external drainage tube (C). Three months after operation, CT showed gross ascites causing scalloping of liver margins, without intrahepatic bile duct dilation (D)
Follow-up
Patients were followed up after the procedure and subsequent factors were recorded: treatment-related toxicity, bilirubin and tumor markers, biliary obstruction-free time, and survival time. Treatment-related toxicity was assessed according to the National Cancer Institute’s Common Terminology Criteria for adverse events (CTCAE, version 4.0) [6]. Imaging and laboratory tests were performed preoperatively and at week 1, and 1- and 3-months post-operation. Drainage tube occlusion was considered to be present in patients who developed symptoms of obstructive jaundice again, and these patients were followed by cholangiopancreatography under fluoroscopic guidance. Overall survival (OS) was calculated from the day of drainage tube implantation to the day of death.
Statistical analysis
Categorical data were described by number (%), while continuous data were expressed as mean ± standard deviation (SD), or median with either minimum or maximum. Categorical data were compared via Pearson χ2 test or Fisher’s exact test, if more than 20% of cells with an expected count of less than five were observed. Kolmogorov-Smirnov’s and Shapiro-Wilk tests were used to determine whether the continuous variables were normally distributed. Variables conforming to a normal distribution were compared via Student’s t-test; otherwise, Mann-Whitney U test was used for comparison. Kaplan-Meier method was used to plot the biliary obstruction-free time and survival curves, while the significance between these curves was assessed through the log-rank test. P- values < 0.05 were considered statistically significant. SPSS v23.0 (IBM Corp.) was used for all statistical analyses.
Results
A total of 58 patients were included in this study after application of appropriate exclusion criteria. The general demographic information of patients is provided in Table 1.
Table 1
Demographic data and clinical profiles of both groups of patients with malignant obstructive jaundice
The technical success rate in the two groups was 100%. Treatment-related toxicity did not significantly differ between the two groups (p > 0.05) (Table 2). Grade 1/2 adverse events (AEs) were relatively mild and alleviated spontaneously or after conservative management. We observed an improvement of grade 3/4 AEs after medication for biliary tract infections increased serum amylase and cardio-biliary reflexes (triggered by pain in the gallbladder via autonomic vagal innervations). Skin leaks, liver abscesses (through direct contact of infected biliary tract), and catheter migration were independently managed by replacement of drainage tubes, percutaneous puncture, and drainage tube adjustments. In addition, 125I stranded seeds migration and 125I seeds dislocation did not appear during follow-up in group A. In all patients, the cause of death was due to tumor progression.
Table 2
Treatment-related toxic events in both groups
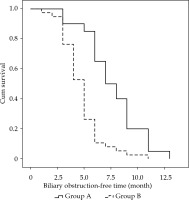
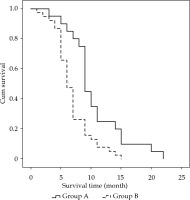
In group A, out of 20 patients, 13 underwent unilateral implantation with internal-external drainage tubes, and 7 patients underwent bilateral implantation with internal-external drainage tubes. An average of 21.4 ±13.0 (range, 10-40) 125I seeds were implanted, with a mean radioactivity of 12.4 mCi ±7.5 (range, 7.0-23.2 mCi) per patient. Estimated total radiation doses were approximately 50-80 Gy, as determined at specific dose reference points calculated using the treatment planning system (TPS; University of Beijing Aeronautics and Astronautics, Beijing, China). The average procedure time for the implantation of 125I stranded seeds for every patient was 31.5 ±5.6 min (range, 25.0-45.0 min). In group B, out of 38 patients, 25 underwent unilateral implantation with internal-external drainage tubes, and 13 patients underwent bilateral implantation with internal-external drainage tubes. The differences in total bilirubin, direct bilirubin, and indirect bilirubin levels between the two groups were not significant at preoperative time points (p = 0.857, p = 0.719, and p = 0.870, respectively), and at the 1-week post-operative time point, these differences were also not significant (p = 0.259, p = 0.395, and p = 0.145, respectively). However, in 1-month (p = 0.012, p = 0.005, and p = 0.049, respectively) and 3-month post-operatively, group A was lower than group B (p < 0.001, p = 0.001, and p = 0.001, respectively), as shown in Table 3. The differences in carbohydrate antigen 19-9 (CA19-9), cancer antigen 125 (CA125), and carcino-embryonic antigen (CEA) levels between the two groups were not significant at the preoperative time point (p = 0.229, p = 0.116, and p = 0.273, respectively), nor were they significant at the 3-month post-operative time point (p = 0.159, p = 0.342, and p = 0.306, respectively), as presented in Table 4. Median biliary obstruction-free time was significantly different between groups (p < 0.001), with 7.0 months [95%CI: 5.2-8.8 months] for group A and 5.0 months [95%CI: 4.4-5.6 months] for group B (Figure 2). Median patient survival time differed significantly (p = 0.001) and was 9.0 months [95%CI: 8.3-9.7 months] for group A and 6.0 months [95%CI: 5.2-6.8 months] for group B (Figure 3).
Table 3
Changes in the preoperative and post-operative bilirubin levels in the two groups of patients
Table 4
Comparison of tumor marker levels in the two groups before and after the operation
Discussion
Quality of life and survival are often poor in those patients with malignant obstructions of the bile duct occurring due to tumors of the pancreas, gall bladder, bile duct, and liver [1,10]. If they do not receive an aggressive treatment, the median survival time is 185 days [11]. Radical surgery is the main therapy for MBO, but due to the complexity of anatomic structure of the bile duct, and the fact that most patients present at late stages, the majority of patients lose the opportunity for surgery [12]. Thus, the selection of the most appropriate treatment approach for these patients is critically important.
In the past, non-surgical treatments such as PTBD or stent implantation have been employed as a palliative approach to improve bile drainage [13,14]. The use of these approaches is, however, accompanied by a risk of occlusion and is limited in its ability to inhibit tumor growth [15,16]. Radiofrequency ablation (RFA) is currently the most preferred approach to be employed as a first-line palliative intervention in those individuals unable to undergo tumor resection [17]. But when the liver tumor or cholangiocarcinoma that is the source of this obstruction is near the hepatic hilum, it is associated with more complications including infections, peritonitis of the bile duct, and even fatal biliary chest fistulae. Therefore, in these patients, RFA is a less viable treatment option, and other alternatives must be investigated [18]. Previous studies suggest that radio-chemotherapy can effectively treat MBO caused by biliary tract cancers. Chemotherapy can control systemic disease and allows the local effects of radiotherapy to translate into longer survival rates. However, survival benefits are only observed in non-metastatic disease, whilst those with metastatic disease show no benefits [19]. In addition, only a small number of patients received chemotherapy during follow-up due to their poor status or refusal of treatment.
In recent years, brachytherapy has been widely utilized for tumor treatment. It is performed by implanting radioactive sources either directly into the tumor tissue (interstitial brachytherapy) or into hollow organs (intracavitary radiotherapy). Results to date suggest that it is possible to achieve significant efficacy when using this approach to treat portal vein tumor emboli, prostate carcinomas, lung cancer, and MBO in particular [8,20]. Compared to intracavitary radiotherapy, interstitial brachytherapy is restricted for MBO. Due to the location and morphology of the lesions, a uniform distribution of 125I seeds was difficult to achieve at the site of lesion, which may impact its therapeutic effects. Secondly, patients who underwent percutaneous puncture operations were at higher risk. Thirdly, due to operator experience and skills limitations, only interstitial brachytherapy was performed in some centers [13,21,22]. However, intracavitary radiotherapy was feasible due to its minimally invasive technology and ease of manipulation [23]. For MBO, this method offers many advantages. Firstly, the radioactive source is small in size, permitting close proximity to the biliary duct. Secondly, this approach delivers high doses of radiation to localized tumors with limited systemic side effects. Thirdly, this approach represents a form of conformal radiotherapy that is associated with improved accuracy because of patient movement [24]. The mainstay treatment method of intracavitary radiotherapy consists of 125I stranded seeds and irradiation stents, all of which show efficacy. Unfortunately, obvious disadvantages exist for related irradiation stents, particularly when stent dysfunction is impossible to avoid. The exact causes of stent dysfunction were unclear. In addition, irradiation stents cannot be immediately withdrawn in any instance, as intracavitary brachytherapy-related complications can occur [6,24].
In our department, 58 patients diagnosed with MBO were enrolled in this study. In group A, 20 patients underwent PTBD combined with 125I stranded seeds, whereas in group B, 38 patients underwent simple PTBD. Technical success was achieved in all patients. Post-operation, the observed toxicities in the two groups showed no significant differences. Grade 1/2 AEs alleviated spontaneously or after conservative management. Grade 3/4 AEs including biliary tract infections, increased serum amylase, and cardio-biliary reflexes improved after medication. Skin leaks, liver abscesses, and catheter migration were independently managed by replacement of drainage tubes, percutaneous puncture, and drainage tube adjustments. In addition, 125I stranded seeds migration and 125I seeds dislocation did not appear during follow-up in group A. Iodine-125 is a low-energy radioisotope that is sufficient to provide a robust local radiation dose and good curative effect while causing minimal extraneous damage. This was a result of the effective range of 125I seeds being ~1.7 cm, such that surrounding normal tissue only received ≤ 25% of radioactive dose received by tumor cells [14,25].
The increased bilirubin observed after PTBD may be due to tumor ingrowth, tissue hyperplasia, sludge formation, or secondary biliary cirrhosis [14,26]. The observed reduction in bilirubin in group A was more pronounced than group B, with the likely explanation being that the patients in group A had undergone implantation with 125I seeds. This implantation provided sustained localized irradiation to inhibit tumor growth and tissue hyperplasia, achieving long-term maintenance of normal bilirubin levels [14]. The excretion of bile was not itself affected by the 125I stranded seeds.
The term “tumor marker” refers to the production of bioactive substances by malignant tumor cells that are then released into the blood. Serum levels of tumor markers can assist tumor diagnosis and evaluation of therapeutic effects of treatment [27,28]. In this study, preoperative and 3-month post-operative tumor marker levels did not significantly differ between the groups. There are several possible explanations for these findings. First, in group A, after 125I stranded seed implantation, tumor cells sustain damage, and tumor degradation leads to a release of tumor markers into the blood [29], which could be another possible explanation is the delay in achieving steady-state levels following 125I seed treatment [30]. Thus, in this study, no differences in the tumor marker levels between the two groups of patients were observed.
The median biliary obstruction-free time was 7.0 months for group A and 5.0 months for group B (p < 0.001). In group A, continuous low energy intracavitary brachytherapy can inhibit tumor mitosis, causing the tumor cells to remain in the G2 phase, resulting in eventual tumor cell killing by radiation. This approach can prevent tumor growth into the lumen of internal-external drainage tube, thereby reducing the risk of internal-external drainage tube stenosis or occlusion, as compared to the drainage alone [31,32]. Due to the long biliary obstruction-free time, this approach also reduces the frequency, at which patients undergo an exchange of internal-external drainage tubes and hospital stay, in addition to the cost of treatment.
The median survival time was 9.0 months for group A and 6.0 months for group B (p = 0.001). In addition, the survival times in group A showed a promising outcome compared to conventional drainage alone, as observed by Zhang et al. [12]. In group A, the 125I stranded seeds were used as the radioactive source of intracavitary brachytherapy for the treatment of MBO. Iodine-125 is a synthetic radioisotope of iodine allowing for the delivery of γ-ray radiation using radioactive seeds. Through γ-ray-mediated induction of tumor cell cycle arrest and inhibition of tumor angiogenesis, the goal of suppressing bile duct endothelial hyperplasia and disrupting the growth of cancerous cells are possible to achieve [33,34,35,36]. In addition, 125I seeds deliver sustained irradiation to the tumor and can further oxygenize hypoxic cells, causing the tumor to become more sensitive to intracavitary brachytherapy, thus enhancing treatment efficacy [14]. Additionally, 125I stranded seeds implantation offers the advantage of extended period of remission for those suffering from obstructive jaundice, thereby improving patient liver function and performance, delaying disease progression, and prolonging overall survival [31]. Moreover, the 125I stranded seeds can be exchanged to further control tumor growth [36]. As such, a combination of drainage and 125I stranded seed implantation compared to drainage alone can prolong survival times in patients with MBO.
This study has several limitations. Firstly, the sample size was not large enough to obtain a powerful statistical conclusion regarding the biliary obstruction-free period or overall survival. Due to this limited sample size, results may be influenced by the population distribution of the two groups in a manner unrelated to the 125I stranded seeds themselves. Secondly, a prospective, randomized, multicenter study is needed to clarify the effectiveness of 125I stranded seeds for the treatment of malignant biliary obstruction. Thirdly, a skin surface dosimeter was not employed after 125I stranded seed placement; therefore, the precise radiation dose delivered to every patient remain unknown.
Conclusions
In summary, intracavitary brachytherapy may offer benefits to prolong the survival of patients with MBO. Iodine-125 stranded seeds can be implanted along the internal-external drainage tube inserted at the site of a given lesion through a simple operation. It can more effectively restrain the proliferation of endothelial cells in the bile duct and disrupt the growth of cancerous cells. This approach is therefore worthy of further investigation and utilization in clinical practice.