Introduction
Non-transparent lesions measuring ≤ 3 cm in diameter on imaging that are completely encased by lung parenchyma are considered pulmonary nodules (PNs) [1–3]. Malignant neoplasms and benign PNs have been previously described [1–3]. When the diameter of the PNs is between 6 and 8 mm, frequent computed tomography (CT) follow-up is required, as recommended by the Fleischner Society recommendations [4]. A repeat CT scan in 3 months, positron emission tomography (PET)/CT, or a tissue biopsy should be considered for PNs with a diameter more than 8 mm [4]. Currently, CT-guided biopsy is most often utilized for PN diagnosis [5–7]. However, CT-guided biopsy, in contrast to CT screening, needed recurrent scanning throughout the biopsy operation, and hence may be linked with much greater radiation doses compared to routine diagnostic CT imaging approaches [8–10].
Many studies have employed low-dose CT for biopsy guidance in order to reduce radiation exposure to PNs [11–16]. Compared to the normal-dose CT guidance, the radiation dose can be reduced by 81–92% by using low-dose CT, without reducing the overall diagnostic accuracy [11–16]. Although major dose reduction can exacerbate noise and decreased image quality, use of iterative reconstruction technique can significantly reduce the image noise and provide better overall image quality [15]. However, owing to variations in research design, CT equipment, scanning settings, biopsy needle types, or operator expertise, outcomes may vary widely from study to study. A convincing conclusion about low-dose CT-driven biopsy for PNs requires a meta-analysis.
Aim
The purpose of this meta-analysis is to evaluate the efficacy and safety of low-dose CT-guided biopsy in diagnosing PNs.
Material and methods
This study has been registered at INPLASY.COM (No. INPLASY202370073) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed when this meta-analysis was conducted.
Study selection
Up to June 2023, we used the following formula to search PubMed, Web of Science, and Wanfang for relevant studies: (((((computed tomography) OR (CT)) AND ((lung) OR (pulmonary))) AND (nodule)) AND (biopsy)) AND (low-dose). Studies needed to satisfy the following requirements to be included: (a) studies using comparisons, (b) patients with PNs, (c) therapies involving low- vs normal-dose CT-guided biopsy, and (d) languages not otherwise specified. Studies were not included if they met any of the following criteria: (a) they were not comparative, (b) their titles and/or abstracts were not written in English, and (c) they were case reports, letters, or reviews.
Data extraction
Two writers independently retrieved relevant data from eligible papers, and disagreements were settled by discussion with a third writer. The number of patients, their ages and gender ratios, the diameter of their PNs, and the distance between their PNs and the pleura were all extracted as well as the study’s first author, publication year, nation, study design, and methodology. The rates of pneumothorax and pulmonary bleeding, as well as the frequency with which these complications necessitated insertion of a chest tube, were among the extracted outcome data.
Quality assessment
Randomized controlled trials (RCTs) were evaluated for quality using the Cochrane risk-of-bias methodology. RCTs were classified as high, low, or uncertain quality based on their detection, performance, selection, attrition, reporting, and other types of bias.
The quality of studies that were not RCTs was evaluated using the Newcastle-Ottawa scale (NOS), which ranks studies based on their comparability (2 points), selection (4 points), and outcomes (3 points). Research quality was judged to be excellent if the NOS score was ≥ 7.
Endpoints
The dose-length product (DLP) value is the major outcome measure in this meta-analysis, with secondary outcomes including diagnostic yield, diagnostic accuracy, biopsy process length, pneumothorax rate, pulmonary bleeding rate, and the rate at which complications necessitated chest tube insertion. No specific radiation dose has yet been set for low-dose CT, but some researchers have suggested that the effective dose of low-dose CT is < 2.5 mSv [17]. The yield of a diagnosis was defined as the ratio of cases with biopsy-based benignity plus malignancy to the total number of included patients. True plus false positives divided by the number of patients who were given a definitive diagnosis was utilized for estimating the diagnostic accuracy [15]. The dose-length product was used to calculate the exposure level.
Statistical analysis
Stata v12.0 and RevMan v5.3 were utilized for the combined analysis. Mean difference (MD) values with 95% confidence intervals (CIs) were used for comparisons of continuous variables while 95% CIs and pooled odds ratios (ORs) were computed for dichotomous variables. Heterogeneity was evaluated using the I2 statistic and Q test, with a value of I2 > 50% indicating substantial heterogeneity. When the degree of heterogeneity was high, random-effects models were employed, while fixed-effect models were applied otherwise. In an attempt to identify the origins of the observed variation, sensitivity analyses were performed using a “leave one out” strategy. Historically, funnel plots were used to examine potential publication bias. Publication bias would be minimal if all research fell within the narrow part of the funnel plots. Otherwise, the article was evaluated using Egger’s test with a significance level of p < 0.05.
Results
Included studies
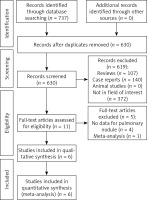
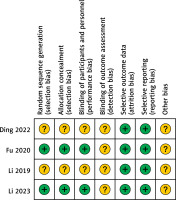
A total of 737 studies were found that fit the criteria of the study plan. After careful screening, we included 6 studies in our meta-analysis (Table I). Figure 1 depicts the methodology used to identify studies. There were four randomized controlled trials and two retrospective investigations. The included investigations were only from China. Two out of the four RCTs have an ambiguous risk of bias in selection, performance, detection, and others (Figure 2), while the other two have an ambiguous risk of bias in detection and others. The NOS of 8 is shown in both of the retrospective investigations.
Table I
Baseline data of the included studies
| No. | First author | Year | Country | Design | NOS |
|---|---|---|---|---|---|
| 1 | Ding [11] | 2022 | China | Randomized controlled trial | – |
| 2 | Fu [12] | 2020 | China | Randomized controlled trial | – |
| 3 | Huang [13] | 2021 | China | Retrospective | 8 |
| 4 | Li [14] | 2019 | China | Randomized controlled trial | – |
| 5 | Li [15] | 2023 | China | Randomized controlled trial | – |
| 6 | Zhang [16] | 2021 | China | Retrospective | 8 |
Low-dose CT-guided PN biopsies were performed on 459 patients while normal-dose CT-guided PN biopsies were performed on 384 patients throughout the 6 investigations (Table II). Table III displays the specifics of the CT apparatus, parameters, and biopsy needle parameters.
Table II
Characteristics of the patients in the included studies
| Studies | Groups | Patients (n) | Age [years] | Gender (M/F) | BMI [kg/m2] | Nodule diameter [mm] | Lesion-pleura distant [mm] |
|---|---|---|---|---|---|---|---|
| Ding [11] | Low-dose | 30 | 37–68 for all | 29/31 for all | Not given | Not given | Not given |
| Normal-dose | 30 | Not given | Not given | Not given | |||
| Fu [12] | Low-dose | 52 | Not given | Not given | Not given | 22.2 | Not given |
| Normal-dose | 50 | Not given | Not given | Not given | 20.8 | Not given | |
| Huang [13] | Low-dose | 70 | 63.5 | 45/25 | 23.1 | 24.7 | 17 |
| Normal-dose | 65 | 59.9 | 39/26 | 22.9 | 23.6 | 20 | |
| Li [14] | Low-dose | 140 | 61 | 97/43 | 27.1 | Not given | 23 |
| Normal-dose | 70 | 66 | 44/26 | 26.4 | Not given | 22 | |
| Li [15] | Low-dose | 100 | 63.7 | 68/32 | 23.1 | 24.8 | Not given |
| Normal-dose | 100 | 61.1 | 62/38 | 22.8 | 23.5 | Not given | |
| Zhang [16] | Low-dose | 67 | 63.4 | 44/23 | 23.2 | 24.6 | 16.4 |
| Normal-dose | 69 | 60.2 | 41/28 | 23.0 | 23.6 | 20.3 |
Table III
Characteristics of the CT and biopsy needles in the included studies
| Studies | CT types | CT manufacturers | Scanning parameters | Needle types | Needle size | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low-dose group | Normal-dose group | |||||||||
| Tube voltage [kV] | Tube current | Thickness [mm] | Tube voltage [kV] | Tube current | Thickness [mm] | |||||
| Ding [11] | 64 row | Siemens | 120 | 40 mA | 1 | 120 | 120 mA | 1 | Core needle | 18G |
| Fu [12] | 16 row | Philips | 120 | 15 mAs | 2 | 120 | 150 mAs | 2 | Core needle | 18G |
| Huang [13] | 16 row | Philips | 120 | 15 mAs | 2 | 120 | 150 mAs | 2 | Core needle | 18G |
| Li [14] | DSCT | Siemens | 100 Sn | 70 mAs | 4 | 110 | 50 mAs | 4 | Core needle | 18G |
| Li [15] | 16 row | Philips | 120 | 15 mA | 2 | 120 | 150 mA | 2 | Core needle | 18G |
| Zhang [16] | 16 row | Philips | 120 | 15 mAs | 2 | 120 | 150 mAs | 2 | Core needle | 18G |
Diagnostic yield
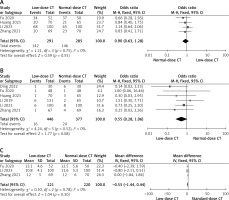
Diagnostic yield rates were reported in four trials [12, 13, 15, 16], with a total of 291 patients undergoing low-dose CT-guided biopsy and 285 undergoing standard-dose CT-guided biopsy. Low-dose and normal-dose groups show no remarkable heterogeneity (I2 = 0%, Figure 3 A) in their pooled diagnostic yield rates, which are similar at 48.8% and 51.2%, respectively (p = 0.55).
Diagnostic accuracy
The diagnostic accuracy rates were given in all investigations, and the pooled rates show no significant heterogeneity (I2 = 0%) between the low- and normal-dose groups (96.4 vs. 93.6, p = 0.08, Figure 3 B).
Biopsy procedure duration
There were 3 studies that looked at how long the biopsy process took, reporting on 221 patients who had a low-dose CT-guided biopsy and 220 patients who had a normal-dose CT-guided biopsy [12, 15, 16]. Neither the low- nor the normal-dose groups showed any significant heterogeneity (I2 = 0%) in the pooled biopsy procedure time (MD = –0.50, p = 0.30, Figure 3 C).
DLP
The pooled DLP shows a remarkable difference between the low- and normal-dose groups (MD = –359.28, p = 0.01, Figure 3 D). All studies reported the DLP value. Nonetheless, the heterogeneity is noteworthy (I2 = 100%). The origin of the variation was not determined by the sensitivity analysis.
Pneumothorax
The rates of pneumothorax were reported in five studies [12–16], with a total of 431 patients undergoing low-dose CT-guided biopsy and 355 undergoing standard-dose CT-guided biopsy. There is no significant heterogeneity (I2 = 0%) between the low- and normal-dose groups in the pooled pneumothorax rates (16.5% vs. 17.2%, p = 0.61, Figure 3 E).
Pulmonary hemorrhage
Pulmonary hemorrhage rates have been reported in five investigations, including 431 and 335 patients who had low- and standard-dose CT-guided biopsy, respectively [12–16]. Comparison of the pooled rates of pulmonary bleeding between the low-dose and normal-dose groups reveals no remarkable heterogeneity (I2 = 0%; 14.8% vs. 13.5%, p = 0.29, Figure 3 F).
Complications requiring chest tube insertion
Low-dose CT-guided biopsy was studied in two investigations [14, 15], with 240 patients and normal-dose CT-guided biopsy in one study each, reporting on the frequencies of complications requiring chest tube insertion. Without any remarkable heterogeneity (I2 = 0%), the pooled rates of complications requiring chest tube insertion are similar across the low-dose and normal-dose groups (3.3% vs. 2.4%, p = 0.48, Figure 3 G).
Publication bias
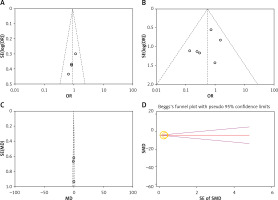
The funnel plots showed that there is no significant risk of publication bias in the diagnostic yield, diagnostic accuracy, pneumothorax rate, pulmonary hemorrhage rate, and the rate of complication required chest tube insertion (Figures 4 A–G). Egger’s test showed that the risk of publication bias of biopsy procedure duration was low (p = 0.999), while the risk of publication bias of DLP was high (p = 0.034).
Discussion
This meta-analysis compared low- to standard CT-driven PN biopsy to evaluate its diagnostic efficacy and safety. No remarkable discrepancy in the diagnostic yield or accuracy rates between the low- and normal-dose groups was found, suggesting that the former procedure does not compromise the accuracy with which CT-driven PN biopsy could diagnose patients. CT-guided PN biopsy often does not need fine lesion details, but suitably viewable positions of the needle lesion and tip are required [18]. Despite the inferior imaging quality of low- compared to normal-dose CT, this technique is nonetheless widely utilized.
Overall diagnostic accuracy is 95%, with a pooled accuracy of 96.4% from the low-dose group and 93.6% from the normal-dose one. This finding is consistent with previous research on CT-guided pulmonary biopsies [18, 19]. The diagnostic accuracy of CT-guided percutaneous coaxial core needle biopsy of lung lesions is affected by benign pulmonary lesions, pulmonary lesions smaller than 1.5 cm (which provide technical difficulties), and pulmonary lesions bigger than 5 cm (which are associated with a greater necrosis rate) [18]. To improve diagnostic accuracy during CT-guided PN biopsy operations, current practices favor the use of multi-planar reconstruction and co-axial approaches [18, 20]. Using a co-axial approach and multi-planar reconstruction, researchers can more accurately track the needle’s apex.
Moreover, DLP is shown to be considerably lower in the low- than the normal-dose group. The random-effects model nevertheless showed a significant difference between the two groups, despite the high heterogeneity that was also found. As a result, we think that low-dose CT is an excellent way to protect patients from unnecessary radiation. The wide discrepancy might be due to the fact that each study used a different CT setup and related proceedings.
In this systematic review, we found no evidence that using low-dose CT compromises the safety of CT-guided PN biopsy. Chest tube insertion due to complications was also uncommon, with a pooled incidence of less than 5% across both groups in our meta-analysis. Longer needle paths and greater distances between the lesion and the pleura are also associated with an increased risk of pneumothorax and pulmonary bleeding during CT-guided biopsy [21, 22]. Careful evaluation of preoperative CT imaging is necessary for selecting an optimum approach for puncturing the deeper PNs.
There are limitations in this meta-analysis. First, all investigations were conducted in China. Therefore, the global representativeness of this meta-analysis is low. More research from all across the globe is required. Second, the CT parameters used in this investigation were not uniform, which might have led to a high risk of bias. Thirdly, although CT-driven biopsy for PNs is the primary focus of our meta-analysis, not all studies reported the average width of PNs or the average distance from the lesion to the pleura. Therefore, there is a significant possibility of bias in this meta-analysis due to these results.