Purpose
Within the UK, cervical cancer is the 14th most common female cancer, with approximately 3,200 new cases annually [1]. At our institution, we treat cervical cancer with external beam radiotherapy (EBRT), concomitant chemotherapy, and image-guided brachytherapy (IGBT). Before the COVID-19 pandemic, we started brachytherapy during week five or six of EBRT, giving one fraction a week for three to four weeks [2]. During the pandemic, with availability of the anesthetist, anesthetic practitioner, and post-anesthetic care unit (PACU) staff limited, we had to be more resourceful with their availability and our theatre timetable. We changed our practice to support more limited staff numbers and treated two fractions in one day (with a six-hour gap between fractions) following one applicator insertion, thus utilizing one administration of anesthesia.
Previously, the removal of brachytherapy applicators was performed while the patient was still experiencing analgesia from neuraxial (spinal) anesthetic. As we moved to treating two fractions in one session, we noticed patients were in more pain after anesthesia had worn off. Pain and anxiety related to IGBT treatments is a well-recognized experience among patients [3, 4]. Our pain management plan was to use oral and intravenous analgesics as well as inhaled nitrous oxide (Entonox). However, this strategy did not seem to provide a sufficient level of pain relief. We also explored the use of epidural placement with patient-controlled top-ups, but this procedure took longer to perform and required additional nursing and medical support in the intra-fraction period, which was not always possible during the pandemic.
The use of inhaled methoxyflurane (IMF; Penthrox™, Galen Pharmaceuticals) has been well established in the acute trauma and emergency department (ED) setting [5]. It reduces the pain scores of trauma patients and decreases the patients’ length of stay in the ED [6]. In Australasia [7, 8], the use of IMF is described in patients with pain associated with planned procedures rather than acute trauma, including change of burns dressings, incision and drainage of abscesses, and colonoscopies. With knowledge of its previous success, we introduced the IMF inhaler for our gynecological patients undergoing brachytherapy applicator removal. This study was the first to directly assess the impact of IMF introduction in IGBT patients having treatment for gynecological cancers.
Material and methods
Patient pathway
Pre- and post-pandemic patients follow the same previously described initial pathway [2]. The patient is admitted to the day surgery unit (DSU) before transfer to the brachytherapy suite. Patients receive a neuraxial (spinal) anesthetic with diamorphine and 0.5% hyperbaric bupivacaine. The use of diamorphine enhances spinal analgesic effect for up to 12 hours, while the paralyzing effect lasts approximately 4 hours. Intravenous sedation with propofol is then commenced using a target-controlled infusion (TCI) pump. The clinical oncologists insert the applicators under trans-abdominal and/or trans-rectal ultrasound guidance. The applicators available are Utrecht tandem and ovoids, or Venezia applicator (Elekta, Stockholm, Sweden), with addition of interstitial needles as required [9]. Once the applicators are in situ, the patient is transferred to CT scanner whilst still under sedation, the propofol infusion is terminated after the CT scan has confirmed that the applicator is well-positioned and the packing is in satisfactory position. Once recovered, the patient is transferred to the MRI scanner. Target definition and brachytherapy planning is then performed, and fraction one is delivered. Pre-pandemic, the applicator would be removed at this stage, approximately 4 hours after the placement of neuraxial anesthesia, at which time the anesthetic and analgesic effects were still active.
Post-pandemic, the patient returns to the recovery bay for a six-hour gap until delivery of fraction two. During this six-hour gap, the patient remains lying flat and supine. We were concerned that this could be a painful experience for the patient, as their neuraxial anaesthetic wore off. Intravenous (IV) analgesia was prescribed and given at patient request, and also half an hour prior to applicator removal. Analgesic agents available were IV paracetamol or IV morphine. After administration of fraction two, the brachytherapy applicator was removed. The patient was offered further intravenous analgesia and inhaled nitrous oxide during this time. The patient then returned to the ward and could go home that evening. We noted that the time when the pain appeared to be highest was at the point of applicator removal, and the inhaled nitrous oxide did not seem particularly effective.
Introduction of inhaled methoxyflurane
Inhaled methoxyflurane is licensed for use for moderate to severe pain associated with trauma (under close medical supervision). This is generally interpreted as trauma in the ED/acute injury setting. Our institution was using this in the ED for pain associated with traumatic injuries. Therefore, we sought approval from the hospital Drugs and Therapeutics Committee (DTC) to use IMF in a setting that was potentially outside its license. This is a local approval process outside the marketing authority. In order to obtain approval, we presented a service evaluation to the committee to explain the current process, and why it was no longer suitable for our service. We produced evidence of the IMF inhaler being used for similar treatments in Australasia [7], although this would be the first use of this kind in the United Kingdom. Training was provided by the manufacturer via online video conferencing due to the COVID-19 pandemic. We were provided with sample kits containing an inert substance to practice and to train others with this device. There is an online module for further training, which provide staff with certification of training [10]. This allowed for cascade training to occur. Through our internal quality assurance processes, we were required to collect patient experience data following the introduction of the drug, and present to the DTC after six month interval.
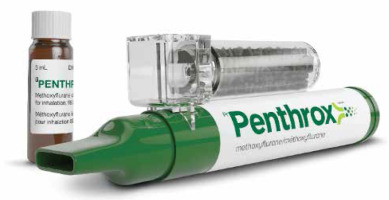
The Penthrox preparation consists of a vial containing 3 ml of 99.9% methoxyflurane with a handheld inhalation device (Figure 1). A maximum of two inhalers or 6 ml of methoxyflurane can be used in a 24 hour period. Administration on consecutive days is not recommended, and the total weekly dose should not exceed 15 ml [11]. IMF cannot be used in patients at risk of renal impairment, and should be applied with caution in cases of hepatic impairment. Manufacturers’ recommendations should be reviewed to check for any other contraindications in individual patients. It is a cost-effective, prescription-only medication, and is not a controlled drug in the UK. The patient is given an explanation of the IMF inhaler and the process of the procedure at a separate appointment several days prior to brachytherapy procedure; a sample inhaler is shown to the patient at this point. At the time of applicator removal, a dedicated radiographer would stand with the patient to remind them of the IMF procedure. A loop is placed around the patient’s wrist, and the patient holds the device and places it in their mouth. Then, the patient breathes through the inhaler for2 minutes, after which time the anesthesia is working. The patient continues to breathe through the inhaler during the procedure, and can increase the strength of the inhaled anesthesia by placing a finger over a small hole in the inhaler. At the end of the procedure, the device is removed and the anesthetic effect wears off within minutes. No formal recovery process is required. The initial 3 ml of methoxyflurane lasts 25-30 minutes of continuous inhalation, which is sufficient for gynecologic brachytherapy applicator removal that usually takes 5-10 minutes, but can take slightly longer if the patient experiences bleeding and requires placement of hemostatic agents or vaginal packing.
Patient experience assessment
We gained retrospective data on patients’ experience and pain scores by sending questionnaires by post to patients who had received two fractions of brachytherapy in one day in the period before IMF was introduced. These were sent 2 weeks to 4 months after the procedure. The purpose of the questionnaires was to collect data regarding pain experienced throughout different stages of the procedure, including pre-procedure, peri-procedure, and post procedure. We used a scale from zero (no pain) to ten (extreme pain). The questionnaire also gathered data on patients’ anxiety post-treatment regarding their next treatment, and if this was heightened due to the pain they experienced. We used the same retrospective questionnaire to assess pain scores in patients who used IMF. These were sent 2-4 weeks after the brachytherapy procedure. These questionnaires were considered part of service improvement; therefore, it did not require ethical approval to send out to patients. A prospective ten-point pain scale was used to assess pain after applicator insertion. Once the patient had recovered from sedation, the pain scale was filled in at hourly intervals. Pain scoring was omitted if the patient was sleeping or did not want to answer. This was considered part of the patients’ routine care and ethical approval was not required for the retrospective review. Student’s t-test was used to assess significance for the retrospective data collection.
Results
We received retrospective questionnaire feedback from thirteen patients who had treatment before IMF was introduced, and seven patients after IMF was introduced. A median score of six (range, 0-10) was observed for patients’ anxiety, and zero (range, 0-10) for patients’ pain prior to the brachytherapy procedure. After IMF introduction, a significant decrease in median pain score was noted during applicator removal, and one hour after applicator removal (Table 1). Prior to IMF introduction, 4/11 (36%) patients were anxious about their second insertion following the first brachytherapy, two patients did not respond to this question. The rates of anxiety were similar at 2/7 (29%) following IMF introduction. The median score for comfort for one insertion between brachytherapy fractions was 5 (range 0-10) pre-IMF, and 1 (range 0-5) post-IMF (p = 0.1). The majority of patients did not require additional pain relief at home after the procedure (65%).
Table 1
Retrospective pain scores in patients following brachytherapy applicator placement and removal
During the prospective evaluation period after the introduction of IMF, 117 brachytherapy insertions were performed using neuraxial anesthesia and IV conscious sedation during delivery of 177 brachytherapy treatments. Patients who had brachytherapy for other cancers, such as endometrial or vulval cancer, were excluded. Data from 6 insertions (5 patients) were excluded because patients did not use IMF. Four patients were excluded due to no pain score data being collected, and one had 2 IMF during the overall procedure time. Therefore, 77 procedures in 44 patients were analyzed. 33 insertions treated a single fraction and 44 insertions treated two fractions in one applicator insertion (Table 2). Pain scores at some data intervals were not documented, for example, if the patient was asleep or did not want to give a score.
Table 2
Prospective evaluation of pain scores during brachytherapy after the introduction of inhaled methoxyflurane
When using prospective evaluation of the pain, it was seen that patients undergoing a single fraction of brachytherapy had a median pain score of 0 (range 0-7) following insertion and during the waiting time prior to treatment. In the two patients that experienced severe pain (score 6 or above) after insertion, it was highest in the hour after the insertion. Immediately prior to applicator removal, the median pain score was 0 (range 0-7), 6/31 (19%) patients reported pain ranging from 1 to 7. The one patient who reported a pain score of 7 was the patient with a pain score of 7 reported 1 hour after treatment, which dropped to 0 following IMF and applicator removal. After applicator removal, the median pain score was 0 (range 0-4), with only 2/32 (6%) patients reporting pain with scores of 2 (decreased from 5) and 4 (increased from 2).
In patients undergoing two fractions of brachytherapy in a single insertion, there was a median pain score of 0 (range, 0-7) following insertion and during the waiting time prior to treatment. The highest pain score was seen 5 hours after insertion. Immediately prior to applicator removal, the median pain score was 0 (range 0-10), 12/28 (43%) patients reported pain at this time point, ranging from 1 to 10. The one patient who reported a pain score of 10 was the patient with a pain score of 7 at five hours after treatment, which dropped to 5 following IMF. After applicator removal, the median pain score was 0 (range, 0-5), with 4/28 (14%) of patients reporting pain with scores of 2 to 5.
Not all patients were able to use the IMF inhaler. Some patients had difficulty inhaling it or disliked the taste of the IMF; however, they usually adjusted to the taste quickly. Some patients developed very restless legs, which could pose a safety challenge when the patient had their legs in stirrups in the lithotomy position. Patients often experienced a euphoria during using the IMF, which some found disorientating, but many found slightly intoxicating, and were enthusiastic about having ‘the green whistle’ on subsequent occasions. A minority of patients experienced marked amnesia regarding the removal process, which was not regarded as a negative event. IMF cannot be used in patients at risk of renal impairment, but all patients have their renal function checked prior to brachytherapy, so this is not a concern in the in-patient setting.
Discussion
These results show a marked decrease in patients’ pain scores when using IMF during brachytherapy applicator removal. This decrease was seen in both prospective pain score measurement and in recollected pain scoring on retrospective questionnaires. Interestingly, this decrease in pain did not lead to a drop in patients’ anxiety prior to the next treatment.
Initial introduction of methoxyflurane as an anesthetic agent was associated with renal damage, therefore, its use was suspended. However, further research showed safety and efficacy of IMF for pain relief when used in small doses in conscious patients. Research demonstrates extensive use of IMF in trauma cases within the ED and pre-hospital setting [5, 12]. As it is a self-administered, fast acting drug with minimal effect on patients vital signs [13], it is optimal for those in significant pain. A randomized study of IMF vs. standard analgesia (comprising intravenous non-steroidal anti-inflammatory drugs escalating to non-opioid and opioid analgesia) following acute trauma demonstrated a 30% improvement in acute pain for 86% of IMF patients vs. 58% of standard analgesia patients [14], with a median time to first pain relief of 3 minutes for the IMF cohort. Similar analgesic results were seen in the ‘STOP!’ trial, with a median time to first pain relief of 5 minutes [15].
The licensed indication for IMF in the UK is for emergency relief of moderate to severe pain in conscious adult patients with trauma and associated pain. The use of IMF in the out-patient setting has been described in Australia and New Zealand [16, 17]. In New Zealand, a small study was performed to assess the efficacy of IMF for a variety of out-patient procedures, including removal of brachytherapy applicators [7]. 123 patients underwent 173 minor surgical procedures with the use of IMF, and a success rate of 97% was documented. Of these 173 procedures, 25 were brachytherapy applicator removal, and the patients were given self-administered IMF with 100% success. IMF being used as an alternative pain relief in an out-patient setting has not been described outside Australasia.
The cervical brachytherapy experience is recognized as very traumatic to patients [18], and is a significant contributor to long-term distress following the use of curative chemo-radiotherapy for cervical cancer [19]. Psychological distress during treatment has an impact on health-related quality of life not only during treatment, but for several months afterwards, and may also effect sexual satisfaction following treatment [20, 21]. We noted high levels of anxiety in our patients prior to the brachytherapy experience, despite active interventions aimed at decreasing anxiety. Pain relief during brachytherapy has been identified as a challenge for many years, and pain can result in sub-optimal applicator placement [3]. Prior to the introduction of IGBT, intravenous conscious sedation can be used throughout the procedure to provide excellent pain relief and amnesia [22]. However, at that time the average procedure length was 1.4 hours, whereas with IGBT, the procedure length is at least 3.5 hours [2], and significantly longer when treating two fractions in a day. The use of epidural and spinal analgesia has been described showing excellent analgesic effect [23]. However, during the COVID-19 pandemic, the availability of specialist staff to monitor and maintain an epidural infusion was limited, and the extra time required to place an epidural rather than a spinal anesthetic was often not available. Therefore, the use of IMF allowed patients to complete their brachytherapy treatment with decreased pain and anxiety.
Use of diamorphine in the spinal analgesia is shown to deliver a more prolonged analgesic effect, contributing to the low pain score seen after applicator insertion. Prior to the introduction of IMF, the patient received intravenous morphine and paracetamol half an hour prior to applicator removal. However, the level of analgesia that the spinal diamorphine and intravenous analgesia provided, did not seem adequate for the more traumatic process of applicator removal, particularly at the point when local pressure is applied to the cervix to cause hemostasis. This is when the IMF gave additional pain relief. Also, the IMF often provided a degree of amnesia, so that patients’ recollection of applicator removal was less distinct.
This is the first paper to describe the use of IMF solely in a brachytherapy population. The introduction was combined with retrospective and prospective assessment of pain and anxiety. When assessing pain via a retrospective questionnaire, there is the possibility of recollection bias. Some of the pre-IMF retrospective questionnaires were sent at a longer time interval to the post-IMF retrospective questionnaires; however, it appears that longer time intervals to response do not lead to more favorable recollections [24].
Conclusions
The introduction of IMF successfully delivered short-term pain relief and a decrease in anxiety to women undergoing brachytherapy applicator removal. IMF has enabled patients to undergo two brachytherapy fractions in a day whilst using minimal additional anesthesia resource, providing optimal care during the resource constraints of the COVID-19 pandemic. The effect has been very successful, and our department will continue using IMF to decrease pain and trauma associated with brachytherapy. The department will put further focus on decreasing anxiety prior to brachytherapy through support and education.