Dear Editor,
Lung transplantation is a viable surgical option for patients with end-stage lung disease. It has been performed for patients with end-stage chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, pulmonary hypertension, and cystic fibrosis. It is a major operation, and the donor organ must be screened extensively prior to transplantation.
Patients are at increased risk for pulmonary embolism after transplantation, with some studies suggesting an incidence as high as 27% [1, 2]. Risk factors for developing a pulmonary embolism after lung transplantation include older age, diabetes mellitus, and pneumonia [3]. Vascular compromise can also greatly complicate allograft function and increase mortality. Native lungs have dual blood supply from the bronchial arteries and the pulmonary arteries, which makes pulmonary ischemia from pulmonary embolism less common. However, newly transplanted lungs have not developed collaterals by the bronchial arteries and are at significant risk for pulmonary ischemia after pulmonary embolism [4].
We describe a 54-year-old man who developed a massive saddle embolus after bilateral lung transplantation. The patient had obesity, type 2 dia-betes mellitus, hypertension, chronic kidney disease stage 2, and idiopathic pulmonary fibrosis requiring 10 liters of home oxygen therapy. The patient was thoroughly evaluated and underwent a double lung transplantation utilizing cardiopulmonary bypass for the surgery. The operative course was complicated by bleeding and coagulopathy, which required transfusion of blood products intraoperatively. There was also a significant increase in the peak airway pressures upon attempts at chest closure, which resulted in the decision to leave the chest open. The patient remained intubated and was transported to the intensive care unit in a critical condition. Bronchoscopy was performed for a systematic inspection of the airway and revealed an intact anastomosis with no complications.
The post-operative course was complicated by acute systolic heart failure due to right ventricular dysfunction requiring inotropic support with epinephrine and dobutamine. The patient also developed vasogenic shock requiring both norepinephrine and vasopressin. On post-operative day 2, the patient returned to the operating room for chest washout and closure. He tolerated the procedure but continued to have persistent cardiogenic shock requiring further inotropic support. On post-operative day 6, he developed worsening hypoxia and hypercapnia with persistent hypotension. The patient subsequently deve-loped pulseless electrical activity and advanced cardiac life support was performed. The decision was made to place the patient on emergency veno-arterial extracorporeal membrane oxygenation (ECMO), which stabilized the patient. Heparin was also initiated for anticoagulation while on ECMO. Transesophageal echocardiography (TEE) was performed at the bedside and demonstrated laminar flow in both the left and right pulmonary arteries with moderately reduced right ventricular function.
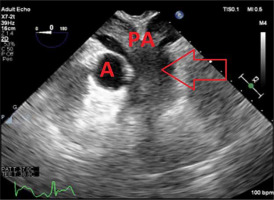
On post-operative day 11, the patient developed persistent bleeding from the ECMO cannulation site. He was taken back to the operating room for ECMO revision. TEE performed intra-operatively revealed a massive saddle embolus and severe biventricular failure (Figure 1).
FIGURE 1
Transesophageal echocardiography 2D image of midesophageal ascending aorta short-axis view showing large saddle embolus spanning the pulmonary arteries. The arrow indicates the large saddle embolus. A – aorta, PA – pulmonary arteries
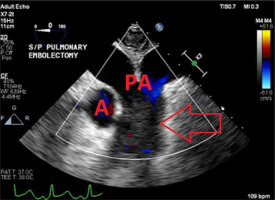
The diagnosis of a saddle embolism was made and a pulmonary embolectomy was performed (Figure 2).
FIGURE 2
Transesophageal echocardiography 2D image of the midesophageal ascending aorta short-axis view after the pulmonary embolectomy. The arrow indicates the patent pulmonary arteries. A – aorta, PA – pulmonary arteries
Unfortunately, the patient continued to require veno-arterial ECMO with maximum inotropic and vasoactive support throughout his hospital course and subsequently died during this admission.
Patients who undergo lung transplantation are at increased risk of developing PTE. In a review of autopsy reports, the incidence of pulmonary embolism in patients who have undergone lung transplantation is around 27% [2]. The etiology for the higher incidence of thromboembolism in lung transplant patients remains unclear though several studies have reported that these patients may be in a hyper-coagulable state with existing co- morbidities [3]. Pulmonary embolism after lung transplantation can significantly increase morbidity and mortality.
The classical signs and symptoms of pulmonary embolism include angina, pleuritic chest discomfort, dyspnea, heart failure, hypoxia, cough, tachycardia, and peripheral vascular compromise. Less commonly patients can present with hemoptysis, exertional syncope, acute cor pulmonale, and cyanosis [5]. However, during the intraoperative and intensive care setting it may be difficult or impossible to recognize some of these symptoms. This highlights the importance of other readily available diagnostic modalities such as TEE.
Regarding our case, TEE performed on post-operative day 6 revealed patent laminar flow in both the left and right pulmonary arteries. It was not until post-operative day 11 that a repeat TEE revealed a massive saddle embolus. This readily available diagnostic tool allowed the physicians to make the appropriate diagnosis and proceed with treatment. Although our patient ultimately died, we believe this case is of value as it demonstrates the utility of TEE in the operative and intensive care setting along with quality images of a saddle embolism as well as return of blood flow after pulmonary embolectomy.
Pulmonary embolism after lung transplantation can significantly increase morbidity and mortality. Patients may not demonstrate the classical signs and symptoms in the perioperative and intensive care setting. It is necessary to include pulmonary embolism in the differential diagnosis in patients after lung transplantation with some of these concerning symptoms. Additionally, physicians should familiarize themselves with TEE images and consider further training with this diagnostic modality. TEE is minimally invasive and readily available in most operating rooms and intensive care settings. This case highlights the utility of TEE for anesthesiologists as well as intensive care physicians and provides impressive images demonstrating a saddle embolism along with return of blood flow after pulmonary embolectomy.




