Introduction
The peritoneum is a thin membrane that covers the walls and organs of the abdominal cavity. Mesothelial cells provide rapid regeneration of the damaged surfaces [1]; however, chronic metabolic disorders affect physiological healing, which leads to adhesion formation [2] and altered permeability of the peritoneal membrane. Not only does the superficial mesothelial layer of the peritoneum suffer from the hyperglycaemia [6] but also the submesothelial connective tissue and vasculature [3].
Although impaired functions of the peritoneum affect its natural defence and integrity in the presence of bacterial peritonitis [4], a paucity of published data exists regarding the impact of the peritoneal infections on the peritoneal morphology in the diabetic population.
We aimed to study the histopathological changes of the peritoneum due to secondary bacterial peritonitis in diabetic rats in comparison to normoglycaemic animals.
Animals
Thirteen intact adult male Wistar rats were used in this study. Their mean age was 9.23 ±1.16 weeks (range 8-11; median 9), and mean weight was 204.77 ±11.3 g (range 190-220; median 206). All animals were quarantined for 30 days before the experiment. Rats were housed in clean laboratory cages with a 12/12 light/dark cycle and followed a standard rat diet. The animals were divided into 2 groups: control (n = 3) and experimental (n = 10). The latter was subdivided into non-diabetic (n = 5) and diabetic (n = 5).
Ethics
The experimental protocol was approved by the local institutional Committee of Bioethics and was in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (Strasbourg, 1986) [5].
Procedures
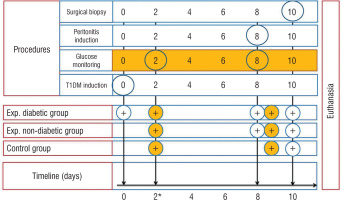
T1DM was induced through a single intraperitoneal injection (IP) of streptozotocin (STZ) – 60 mg/kg (streptozocin, S0130, Sigma-Aldrich) dissolved in ice-cold 0.1 M citrate buffer (pH – 4.5) to 5 rats (experimental diabetic group). The fasting period was 12 hours. Experimental non-diabetic group and controls remained intact. T1DM was verified by measuring the glucose levels using an Accu-Chek Active blood glucose meter (Roche, Germany) 48 hours after injection. Blood glucose level < 14 mmol/l (< 252 mg/dl) was an exclusion criterion. Rats of both experimental groups underwent intraperitoneal injection of 2 ml of faecal matter (2 g of rat faeces mixed with 4 ml of distilled water and filtered with a 4-layer filter) on the 8th day. On the 10th day of the experiment all animals underwent surgical biopsy (Fig. 1). Rats were anesthetized by IP injection of a Ketamine 5% (100 mg/kg) and Xylazine 2% (10 mg/kg) cocktail, and midline laparotomy was performed. For surgical field disinfection, 10% Betadine solution was used. Parietal peritoneum (1 × 1 cm) was collected from the paracolic gutters. In order to collect the sample of visceral peritoneum, ligation of the caecal tip (Polyamide 4/0) and its resection were performed. Macroscopical changes of the peritoneum were noted including colour, plethora of the vessels, peritoneal effusion, etc. The peritoneal cavity was closed hermetically with double row sutures (Polyamide 4/0). All animals were euthanized via anaesthetic overdose (Ketamine 5%, 300 mg/kg) (Fig. 1). For further histopathological studies, the samples underwent the standard procedure of fixation (10% neutral formalin solution), embedding in paraffin and staining with haematoxylin and eosin (H&E).
Results
Three rats of the diabetic experimental group achieved stable hyperglycaemia. The mean glucose level was 21.06 ±3.42 mmol/l and 23.46 ±3.11 mmol/l on the 2nd and 8th day after T1DM induction.
The peritoneal cavity of the controls contains the stomach, liver, spleen, intestine, and gonads. The caecum is a large doughnut-shaped sac. The appendix is not a separate part of the gastro-intestinal tract in rats, but it should be considered as a part of the caecum. The intact parietal rat peritoneum is a bright, pink, thin membrane, which is intimately adherent to the underlying muscles. It contains a significant amount of blood vessels piercing its surface.
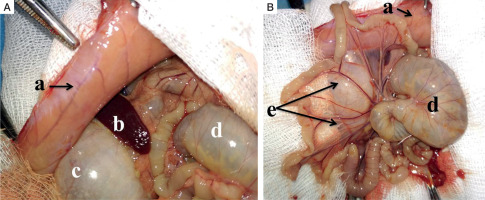
Parietal peritoneum (a) collected from the non-diabetic group 48 hours following the induction of peritoneal infection is moderately oedematous with visible congested vessels. The caecum (d) and stomach (c) are distended (reactive ileus). Mesenterial vessels (e) are congested, and the bowel loops have yellowish discoloration (Fig. 2).
Figure 2
Macroscopic intraoperative view: A, B) adult male Wistar rat, experimental non-diabetic group, peritonitis, 48 hours: a parietal peritoneum, b – spleen, c – stomach, d – cecum, e – mesentery (read description in the text)
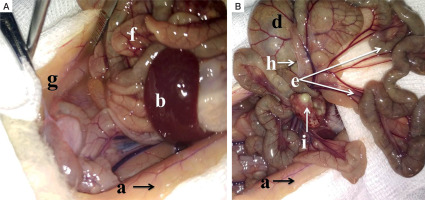
In the diabetic group inflamed parietal peritoneum shows paleness and greyish discoloration. The peritoneal cavity contains interintestinal abscesses (i). Mesenteric vessels are congested. Bowel loops are covered with filmy adhesions (h). Paracolic gutters (g) are filled with the turbid exudate (Fig. 3).
Figure 3
Macroscopic intraoperative view: A, B) adult male Wistar rat, experimental diabetic group, peritonitis, 48 hours: a – parietal peritoneum, b – spleen, d – cecum, e – mesentery, f – bowel loops, g – exudate in the paracolic gutters, h – filmy adhesions, i – interintestinal abscesses (read description in the text)
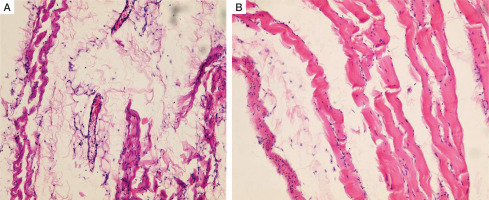
Intact rat peritoneum is a thin lining, which contains a single layer of the flat mesothelial cells, mesothelial basement membrane, superficial tortuous collagenous layer (connective tissue), superficial diffuse and deep longitudinal elastic meshworks, and a deep sleeve elasto-collagenous layer. It is pierced by the collagenous and reticular bundles and is intimately adherent to the underlying muscles (Fig. 4).
Figure 4
Histopathology of the intact parietal peritoneum in an adult male Wistar rat. A) Collagenic and elastic fibres, sporadic histocytes, lymphocytes and fibroblasts, vascular congestion (H&E, ×100). B) Superficial rippled collagenic layer (H&E, ×200)
Peritoneum of the non-diabetic group 48 hours after onset of peritoneal infection presents with degenerative and inflammatory lesions such as fibrin deposits and multiple neutrophilic infiltrates at its surface. Microcirculation is significantly impaired and presents with vascular dilation and congestion, as well as severe oedema of the peritoneum (Fig. 5A).
Figure 5
Histopathology of the peritoneum, adult male Wistar rat (H&E, ×100): A) non-diabetic group, peritonitis 48 hours; B) diabetic group, peritonitis 48 hours
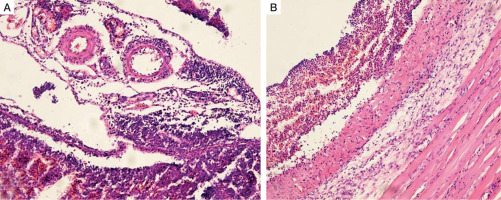
Similarly to the euglycemic rats, significant diffuse degenerative changes and areas of destruction were found in the peritoneum of the experimental diabetic group. Furthermore, subjacent muscular and adipose tissues were affected, and multiple necrotic niduses with abscesses were present in this animal group (Fig. 5B). The surface of the peritoneal lining contained erosions and was covered with oedematous granulation tissue and a thin layer of fibrin. The last one was pierced by destroyed neutrophils. Granulation tissue showed signs of acute exudative inflammation, areas of haemorrhages, and inflammatory cell infiltration.
Discussion
Acute peritoneal infections are associated with direct injury and dysfunction of the peritoneal membrane [6]. Bacterial peritonitis precipitated by T1DM comorbidity is a life-threatening condition. However, we did not find any available histopathological publication on secondary peritonitis in such patients, as well as in animal models. The main limitation is the short-term observation period due to the high mortality of the infected animals [6]. In contrast, a plethora of studies shows features of the long-term aseptic peritoneal damage such as peritoneal fibrosis, neoangiogenesis, and decreased ultrafiltration capacity, especially in patients on peritoneal dialysis [6-8].
We offer a 2-staged mini-invasive technique for both T1DM and bacterial peritonitis induction, in order preserve the intact peritoneum and to monitor its changes due to acute bacterial infection. This approach minimizes risks of iatrogenic intraabdominal organ damage and wound infections following laparotomy [9].
Intraoperative macroscopic findings of the diabetic group included 3-4 ml of turbid foul-smelling fluid collected in the lateral gutters, while non-diabetic rats had less than 1 ml of intraperitoneal exudate. We do not rely on the peritoneal membrane thickness as a cause of exudate accumulation, because short-term hyperglycaemic exposure does not promote severe structural changes of the peritoneal luminal surface [10]. The exact cause of this phenomenon remains unclear; however, functionally impaired permeability and osmotic gradient should be considered as predisposing factors of the excessive intraperitoneal fluid collection.
Kraemer et al. [11] described possible techniques (mechanical and thermal) for adhesion formation in rats with the second look after 14 days. They showed a wide range of adhesions after combined damage of the peritoneum with electrocautery and sutures. We found generalised filmy adhesions in the diabetic group 48 hours after the infection. Forty per cent (2/5) of experimental non-diabetic animals had visible solitary adhesions on laparotomy.
Several studies on peritoneal morphology in rat peritonitis showed the oedematous parietal and the visceral peritoneum, infiltrated by numerous mononuclear cells [12, 13]. It should be noted that the duration of the experiment, the amount of infectious agent, and the means of its administration have an impact on the histological findings. The concentrated faecal matter used in our study caused advanced bacterial peritonitis in all animals 48 hours after the IP injection. Combet et al. described “wound-induced” peritonitis 7 days after the procedure, which showed moderate inflammatory changes of the peritoneum [12].
Conclusions
The natural course of peritoneal infection in diabetic rats (48 hours) is significant for an exudative reaction of the peritoneum in comparison to the non-diabetic ones.
Diabetic rats develop diffuse fibrinopurulent and purulent-necrotic peritonitis with subjacent muscular and adipose tissue involvement 48 hours after the peritoneal infection. In contrast, the same duration of peritoneal infection to the non-diabetic rats causes fibrinopurulent changes of the peritoneal surface while the subjacent tissues remain intact.

 POLSKI
POLSKI