Purpose
Malignant obstructive jaundice (MOJ) is a disorder of bile excretion caused by compression or metastasis of malignant tumors [1]. Clinical manifestations are jaundice of the skin, nausea, itching, dark urine, and discolored stools as well as long-term cholestasis and cholangitis that could cause liver dysfunction and eventually hepatic failure [2]. The European Society of Gastrointestinal Endoscopy (ESGE) strongly recommends the use of self-expandable metal stents (SEMS) for palliative extra-hepatic biliary obstruction (high quality evidence) [3]. Nonetheless, stent re-occlusion caused by tumor growth, overgrowth, connective tissue formation, and sludge formation, affects the application of this technology, increasing the probability of re-intervention and shortening the patency of a stent [4-7]. Studies have found that biliary stents combined with iodine-125 (125I) seed strand implantation might effectively relieve obstructive jaundice and prolong stent patency [8-10]. Even a meta-analysis confirmed the effectiveness of 125I seeds combined with stents [11]. However, the limited number of seeds that could be implanted with this method results in a limited therapeutic effect on primary tumor. As a result, some researchers have attempted to apply percutaneous biliary stenting and computed tomography (CT)-guided 125I seed implantation in the treatment of locally advanced pancreatic carcinoma with concomitant obstructive jaundice, and have found that it may prolong stent patency [12]. We also found that biliary stenting combined with percutaneous or endoscopic ultrasound-guided implantation of 125I seeds into the primary tumor to relieve MOJ, achieved satisfactory results, prolonging patient’s stent patency. To better guide the application of this technology and to identify meaningful clinical application indications, the present study was conducted.
Material and methods
Patients
All patients with MOJ, who had received bile duct stenting combined with 125I seed implantation for the primary tumor from October, 2010 to April, 2022 were enrolled into the study. Patients’ basic clinical information, laboratory and clinical data related to 125I seed implantation and stents, were collected. The study was approved by the Ethics Committee of the Chinese People’s Liberation Army General Hospital (approval code: S2022-464-01), and the requirement for informed consent was waived due to retrospective nature of the study. Inclusion criteria were as follows: (1) Laboratory examinations and imaging studies related to MOJ; (2) Patient receiving bile duct stent placement to relieve obstruction and 125I seed implantation for primary tumor; (3) No chance for R0 resection of primary tumor, or surgery refusal due to personal reasons by patient and family. Exclusion criteria: (1) Patient with benign obstructive jaundice; (2) Patient who received surgical treatment.
Procedure of stent placement and iodine-125 seed implantation
All patients were discussed in the multidisciplinary context before receiving 125I seed implantation, lost the opportunity for surgery, had no indications for radiotherapy and chemotherapy, or refused radiotherapy and chemotherapy for personal reasons. A duodenoscope (TJF-240/TJF-260V; Olympus, Tokyo, Japan) was used to find the duodenal papilla. Cholangiography was performed with X-ray to determine the location and length of stenosis. Then, a plastic stent (Cook Medical, Bloomington, IN) or a fully covered self-expandable metal stent (Boston Scientific, Marlborough, MA) were placed. When the stent was placed under guidewire guidance, the distal end of the stent extended at least 2 cm beyond the stenosis.
Iodine-125 seeds (China Isotope Radiation Co., Ltd., Beijing, China) consisted of a titanium capsule with 125I adsorbed on a passivated palladium rod, which could generate 27.4 keV X-rays with a half-life of 59.4 days were applied. The activity of a single seed was 0.6 mCi. Abdominal CT were used to determine gross tumor volume (GTV) before surgery, and to estimate the number of 125I seeds required. Intravenous or local anesthesia was applied for 125I seed implantation under EUS (Olympus Corporation, Tokyo, Japan) and/or percutaneous ultrasound guidance. Based on our clinical experience, we have adopted a simpler method for seed implantation. Under EUS guidance, a 19 G puncture needle was inserted into the distal edge of the tumor, avoiding blood vessels. Then, the needle core was pulled out and the 125I seed was inserted into the needle. During the withdrawal of the needle core, the seeds were pushed into the tumor tissue to ensure that the distal ends of the two seeds were spaced 1-2 mm apart. The seeds were implanted in a straight line with a density of 5-10 seeds/cm. To prevent the seed from flowing out of the intestinal lumen during needle extraction, the implantation was suspended when the needle was pulled to the outer edge of the intestinal lumen at 2-3 mm. There was no overlap between puncture tracks, that is, the last seed implanted could not be seen during the second puncture. The seeds were made sure to fill evenly throughout the tumor. The number of implanted seeds was accurately recorded. Finally, the position and distribution of 125I seeds in the lesion were evaluated under X-ray. The median number of 125I seeds implanted for each patient for the first time was 30 (range, 10-100 seeds), and the median cumulative radioactivity was 18 mCi (range, 6-60 mCi). The procedure was completed. It should be noted that the detailed procedures have been described in our previous study [13].
Follow-up
All patients’ clinical data were collected by telephone and the Chinese People’s Liberation Army electronic medical record system. The follow-up closing date was April, 2022. Stent patency time was defined as the time from the time the patient received bile duct stent placement, until bile duct obstruction occurred again. If no evidence of bile duct obstruction was found during follow-up and before patient’s death, the patient’s time of death prevailed. In the evaluation of the effect of reducing jaundice at 1 week after the surgery, a decrease in total bilirubin (TBIL) ≥ 50% was defined as a good curative effect, and a decrease in total bilirubin < 50% was identified as a poor curative effect.
Statistical analysis
Statistical analysis software used in this study was SPSS version 25.0. GraphPad Prism version 8.0 was applied to plot the survival curve and forest map, and log-rank test was used to compare the stent patency of the two groups of patients. Cox proportional hazards model was used for the multivariate analysis of stent patency. Univariate and multivariate logistic regression analyses were performed to evaluate factors associated with remission of jaundice at 1 week after operation. For continuous variables, minimum p-value method was applied to determine the optimal cut-off points, and p-value was corrected by approximate correction method (Miller adjustment). Statistically significant difference was indicated by two-tailed p < 0.05.
Results
Basic clinical characteristics, changes in laboratory parameters, and adverse events
As presented in Table 1, a total of 90 patients were enrolled into the study, including 52 males (57.8%) and 38 females (42.2%), with a mean age of 68.66 ±12.53 years (range, 31-90 years). All patients underwent successful placement of bile duct stents and 125I seed implantation. The types of malignancies involved were mainly pancreatic cancer and duodenal papilla cancer. The median stent patency was 8 months. As shown in Table 2, the median (25%, 75%) of TBIL decreased from 152.1 μmol/l (54.4, 300.0) to 66.6 μmol/l (22.0, 145.6), the median (25%, 75%) of direct bilirubin (DBIL) decreased from 117.7 μmol/l (33.8, 239.1) to 49.9 μmol/l (15.9, 99.0), and the median (25%, 75%) of γ-glutamyl transpeptidase (GGT) decreased from 566.8 U/l (326.9, 885.8) to 222 U/l (108.2, 396.8) before and after biliary stent placement, and the difference was statistically significant (p < 0.001). As shown in Table 3, most patients were free of adverse events. The main adverse event was transient abdominal pain (125I seed implantation, 13.3%; biliary stent placement, 16.7%), and a minority of patients had fever (125I seed implantation, 3.3%; biliary stent placement, 4.4%), mild pancreatitis (125I seed implantation, 2.2%; biliary stent placement, 4.4%), and cholangitis (125I seed implantation, 0; biliary stent placement, 4.4%).
Table 1
Basic patients’ characteristics
Table 2
Changes in laboratory results before and after treatment
Table 3
Procedure-related adverse events
Factors associated with stent patency
As shown in Table 4, univariate log-rank test was used to found that bile duct stricture location (high vs. low, 3 vs. 8 months, p = 0.014) (Figure 1A), Child-Pugh score (A vs. B/C, 14 vs. 5 months, p < 0.001) (Figure 1B), biliary infection (yes vs. no, 9 vs. 4 months, p = 0.002) (Figure 1C), biliary stent type (metal vs. plastic, 9 vs. 5 months, p = 0.005) (Figure 1D), ECOG-PS (1 vs. 3, 9 vs. 4 months, p = 0.045) (Figure 1E), pre-operative jaundice duration (≤ 1 month vs. > 1 month, 9 vs. 4 months, p = 0.043) (Figure 1F), and TBIL (≤ 171 umol/l vs. > 171 umol/l, 9 vs. 7 months, p = 0.030) (Figure 1G), could affect stent patency. However, age, sex, pathological type, number of 125I seeds implanted, 125I seed implantation guidance method, pre-operative TP, albumin (ALB), DBIL/TBIL, and GGT had no significant effect on stent patency (p > 0.05). The multivariate analysis showed that Child-Pugh score (HR = 2.221, 95% CI: 1.081-4.562, p = 0.030), biliary infection (HR = 1.901, 95% CI: 1.084-3.335, p = 0.025), and pre-operative jaundice duration (HR = 1.977, 95% CI: 1.106-3.533, p = 0.021) could be used as independent risk factors for stent patency (Figure 2A).
Table 4
Univariate log-rank test of stent patency of bile duct placement combined with iodine-125 seed implantation
Fig. 1
Stent patency curve of different factors (p < 0.05). A) Stent patency curve of different bile duct strictures (low vs. high, 8 months vs. 3 months); B) Stent patency curve of different Child-Pugh (A vs. B-C, 14 months vs. 5 months) C) Stent patency curve with and without bile duct infection (yes vs. no, 8 months vs. 3 months); D) Stent patency curve of different stent types (metal vs. plastic, 9 months vs. 5 months); E) Stent patency curve of different ECOG-PS (1 vs. 2 vs. 3, 9 months vs. 8 months vs. 4 months, 1 vs. 3, p = 0.045); F) Stent patency curve of different jaundice durations before operation (≤ 1 month vs. > 1 month, 9 months vs. 4 months); G) Stent patency curve of different TBIL levels before operation (≤ 171 μmol/l vs. > 171 μmol/l, 9 months vs. 7 months)

Fig. 2
Forest plot of multivariate analysis of biliary stent patency and early jaundice reduction in patients with biliary stent implantation combined with 125I seed implantation. A) Forest plot of multivariate Cox model for stent patency; B) Forest plot of multivariate logistic regression for early jaundice reduction
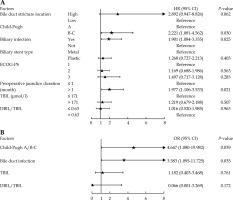
Factors related to the reduction of jaundice at 1 week after operation
According to Table 5, Child-Pugh (OR = 5.858, 95% CI: 1.799-19.082, p = 0.003), bile duct infection (OR = 3.111, 95% CI: 1.113-8.698, p = 0.030), TBIL (OR = 0.376, 95% CI: 0.160-0.885, p = 0.025), and DBIL/TBIL (OR = 0.008, 95% CI: 0.000-0.263, p = 0.007) significantly affected the curative effect of reducing jaundice at 1 week after operation (p < 0.05). Further multivariate logistic regression analysis found that Child-Pugh B/C (OR = 4.647, 95% CI: 1.080-19.982, p = 0.039) and bile duct infection (OR = 3.583, 95% CI: 1.095-11.725, p = 0.035) might be independent risk factors for jaundice reduction at one week after surgery (Figure 2B).
Table 5
Univariate logistic regression analysis of relieving jaundice 1 week after bile duct stent placement combined with iodine-125 seed implantation
Discussion
As an effective palliative treatment for MOJ, bile duct stent placement has been commonly used in clinical practice. However, re-obstruction of the stent increased the probability of re-intervention, shortened the patency of stent, and affected the application of this technology. To effectively prolong stent patency, the conventional stents are constantly being improved. A multicenter study found that an inner conventional uncovered SEMS combined with an outer 125I seed loaded stent could achieve better patency (212 days vs. 104 days) and longer survival (median, 202 days vs. 140 days) than conventional SEMSs [14]. Studies have also found that PTBD combined with iodine-125 stranded seeds seemed to reduce bilirubin levels and prevent biliary obstruction, promoting survival [15]. Another study tried to improve the stent patency in MOJ with radiofrequency ablation combined with a bile duct stent [16]. We have attempted to relieve MOJ by implanting 125I seeds into the primary tumor, inducing biliary obstruction in combination with biliary stents, and found that it also prolonged stent patency. The median stent patency was 8 months in the present study, which effectively prolonged the stent patency compared with stent placement alone (range, 2.8-4.0 months) [9, 17, 18]. In addition, no serious adverse events occurred during follow-up (Table 3).
Stent patency is crucial for patients with advanced MOJ, and longer stent patency might not only reduce the risk of re-intervention, but also reduce the patient’s medical expenses. Evaluating valuable indicators to guide the application of this technology has therefore become crucial. Univariate analysis revealed that the location of bile duct stenosis might significantly affect stent patency. High bile duct obstruction stents have shorter patency than low obstruction (Figure 1A). High bile duct obstruction refers to an obstruction above the opening of the gallbladder duct, especially an obstruction at the hilum, which is common in gallbladder cancer, cholangiocarcinoma, and primary hepato-cellular cancer invading the bile duct [19]. Previous studies have found that stent placement for malignant hilar obstruction was more difficult, and could occlude the side branches of the intra-hepatic bile duct (IHD), especially when more than two stents were deployed, which would reduce the outer sheath diameter and reduce stent patency [20]. In addition, high bile duct obstruction after endoscopic retrograde cholangiopancreatography (ERCP) was more prone to bile duct infection due to difficulty of adequate drainage [21]. Our study found that the bile duct infection might also be a risk factor for the patency of bile duct stents (Table 4). Hence, both, in terms of anatomical structure and pathophysiological mechanism, the stent patency of high biliary obstruction was worse than that of low biliary obstruction. This result was consistent with a previous study on 125I seed strands in relieving MOJ [19]. The Child-Pugh score is a simple and effective tool to assess the condition of patients with cirrhosis [22]. In a study of malignant biliary hilar obstruction, patients’ Child-Pugh scores at the time of stent placement were associated with biliary stent patency [23]. Long-term biliary obstruction, primary tumors, or metastases of the liver could impair liver function. In the Child-Pugh score of liver cirrhosis, grade A was generally in the compensated stage of liver cirrhosis, while grades B/C indicated decompensated stage of cirrhosis. In addition, the number of patients in group C was relatively small, and we included B and C into one group for analysis, based on the above factors. In the current study, we found that Child-Pugh A could achieve longer stent patency than Child-Pugh B/C (Figure 1B). The reason may be that the worse the liver function score in patients with MOJ, the more severe the degree of obstruction, the more severe the tumor progression, and the more serious the damage to the liver. Therefore, we believe that the better the liver function of the patient before surgery, the longer the patient’s stent patency after operation.
During the study, we also found that patients with pre-operative bile duct infection had shorter stent patency (Figure 1C). Bile duct obstruction led to poor bile outflow, which in turn damaged the bile duct epithelial cells and impaired the bile duct immune defense system, which caused bacterial translocation into the bloodstream, leading to infection and sepsis [24]. Moreover, increased production of pro-inflammatory cytokines in patients with bile duct MOJ might also lead to perioperative sepsis [25]. Consequently, effective pre-operative anti-inflammatory treatment for patients with bile duct infection might effectively improve stent patency. In this study, we used conventional plastic stents and metal stents, and found that compared with plastic stents, metal stents combined with 125I seed implantation might achieve longer stent patency (Figure 1D). Plastic stents are generally small in diameter, have a high failure rate, require frequent replacement, and are commonly used as a temporary decompression for palliative MOJ treatment, but metal stents are usually longer and simpler to pass through narrow biliary tracts, with higher technical and clinical success rates, which might prolong stent patency and reduce the need for repeated interventions [20, 26]. Therefore, for MOJ patients with an expected survival of more than 3 months, metal stents are still the first choice. The present study found that compared with uncovered SEMSs, covered SEMSs could better inhibit tumor ingrowth, resulting in longer stent patency [27]. Another study found that partially covered metal stents could achieve longer stent patency compared with fully covered and uncovered metal stents [28]. It is also important to select an appropriate stent placement location. Overlying stents should extend past the blocked area at both ends, otherwise there could be an increased risk of tumor overgrowth at the ends of the stent [4]. In addition, future development of novel tumor-specific biliary stents should broaden the scope of existing treatment strategies, and identify appropriate neoadjuvant therapies to improve long-term survival, develop stent patency, reduce stent migration, and reduce unnecessary re-interventions [29].
The Eastern Cooperative Oncology Group performance score (ECOG-PS) is an important parameter to reflect the physical activity status of oncology patients. In our study, we found that patients with an ECOG-PS score of 3 had poorer stent patency than those with a score of 1 (Figure 1E), which may be related to the poor response of the tumor to 125I seed implantation in patients with a score of 3. In addition, we also found that the duration of bile duct obstruction was more than 1 month, and the stent patency was shorter (Figure 1F). The reason may be that long-term obstruction seriously damage the bile duct and the liver. Timely and effective relief of the obstruction might not only reduce the obstruction symptoms of the patient, but also prolong the stent patency. We also found that for moderate-to-severe jaundice with TBIL > 171 µmol/l, the stent patency was shorter (Figure 1G), which may be due to the higher bilirubin level, more severe bile duct obstruction, and more serious bile duct wall damage, which makes the bile duct more likely to be re-obstructed after operation. Further multivariate analysis found that Child-Pugh B/C, biliary infection, and pre-operative jaundice duration could be used as the independent risk factors for the stent patency.
Iodine-125 seed implantation has been confirmed to be safe and effective in pancreatic cancer [30] and hepato-cellular carcinoma [31, 32]. We have also carried out 125I seed implantation in the clinical treatment of gallbladder cancer and duodenal papillary cancer, and a preliminary clinical effect was achieved. Previous study [13] have confirmed that biliary stents combined with 125I seed implantation in the treatment of primary tumors with bile duct obstruction, might improve the patency of MOJ. The specific mechanism is not yet clear. Although, the placement of the bile duct stent itself could damage the bile duct mucosa, resulting in sub-mucosal inflammation and edema [7], tumor in-growth is a more important factor leading to dysfunction of biliary stents compared with bile duct mucosal hyperplasia or chronic inflammation [33]. We believe that the implantation of 125I seeds might effectively inhibit the growth of tumor tissue, prevent its’ invasion into the bile duct, shrink the tumor, and reduce the external pressure of the bile duct, so as to prolong the patency of bile duct stent in MOJ. Whether biliary stent loaded with 125I seeds combined with 125I seeds implanted into primary tumors might achieve more lasting stent patency, requires further research in the future.
Through the study, it was found that the indicators of obstructive jaundice of the patients decreased significantly one week after the operation, and the clinical symptoms improved. We also found that compared with B/C, Child-Pugh A had a better bilirubin decline at 1 week after surgery (OR = 5.858, 95% CI: 1.799-19.082). In patients with pre-operative biliary infection (OR = 3.111, 95% CI: 1.113-8.698) or with moderate-to-severe elevated TBIL (OR = 0.376, 95% CI: 0.160-0.885) before operation, the effect of reducing jaundice at 1 week after surgery was also poor. In addition, for obstructive jaundice, direct bilirubin was mainly elevated. In our study, we also found that with the increase of DBIL/TBIL, the effect of reducing jaundice at 1 week after surgery was worse (OR = 0.008, 95% CI: 0.000-0.263). However, no significant effect of DBIL/TBIL on stent patency was found, and the specific reasons remain to be further studied. We believe that, although bile duct obstruction is relieved after bile duct stent placement, bilirubin may be quickly decreased, but for patients with moderately-to-severely elevated bilirubin, or with bile duct infection and poor liver function, the reduction of jaundice at 1 week post-operatively may be limited, and may require longer time to return to normal. Through multivariate analysis, it was found that liver function, Child-Pugh B/C, and bile duct infection could not only be independent risk factors for stent patency, but also risk factors for poor reduction of jaundice at 1 week after operation.
It was undeniable that the study had some shortcomings. Since it was a single-center study with a small number of medical records, it was expected that a larger study could be carried out in the future to verify the results. Second, due to the long history of medical records of some patients, detailed clinical data on bile duct stents and bile duct stenosis were not included in the research. Therefore, MOJ patients treated with biliary stents combined with 125I seed implantation, patients with better pre-operative liver function, and those with no biliary tract infection, present not only with longer biliary stent patency, but also a better early jaundice reduction effect.


