Purpose
Treatment of uveal melanoma has been revolutionized since the development of eye-preserving plaque brachytherapy. Different radioactive isotopes have been used as a brachytherapy source for treating these tumors. In 1930, Moore used radon active seeds to treat a case of choroidal melanotic sarcoma. In his report, radon seeds of 1 millicurie strength were embedded in the tumor’s thickest part [1]. Subsequent studies showed an evolution of ocular brachytherapy by introducing different radioisotopes and delivery devices. Nowadays, ocular brachytherapy plaques consist of gold, steel, silver, or titanium shells equipped with either low-energy photon emitter radioactive seeds or beta emitter isotopes. Iodine-125 (125I), palladium-103 (103Pd), and cesium-131 (131Cs) as low-energy photon emitter radioactive seeds, and ruthenium-106 (106Ru) as β emitter radioisotope, have been used as the radioactive plaque sources.
Using 106Ru plaques for ocular melanoma began from primary studies performed by Lommatzsch et al. [2]. The half-life of 106Ru can be six times as high as for 125I [3]. Moreover, the simplicity to implant over the sclera, lower theoretical complication rate, and cost-benefit transportation to distant hospitals from manufacturer country (due to longer half-life) can be considered as the advantages of using 106Ru rather than the other isotopes. On the other hand, lower penetration power into the tumor has created controversies over the use of beta emitter 106Ru in cases of large choroidal melanomas.
The present study aimed to meta-analyze the efficacy of 106Ru brachytherapy to treat choroidal melanoma, review complications, and if applicable, to evaluate the relationship between tumor thickness and local control after brachytherapy with these kinds of plaques.
Material and methods
Our study consisted of 21 peer-reviewed retrospective case series on the efficacy of 106Ru brachytherapy to treat uveal melanoma. PubMed, Embase, Web of Science, Scopus, and Cochrane databases were searched considering medical subject headings (MeSH) thesaurus for the literature published through July 31, 2020. Following key words were applied: “choroidal melanoma”, “uveal melanoma”, “brachytherapy”, and “ruthenium-106” for the title, abstract, and keywords. Following the initial search, 872 papers were selected. Finally, extracted search results were exported to EndNote (Clarivate Analytics, version X7), as a known reference management software, and duplicated records were merged, resulting in 298 indexed papers. Studies presented as reports in meetings and conferences were not included in the review. We applied the following inclusion criteria to extract relevant articles: 1) available English language text, 2) performed on malignant uveal melanoma with or without involving anterior uveal tumors, 3) containing reports about mean or median radiation dose to the apex of the tumor, 4) information about mean or median follow-up time, 5) containing reports on the percentage of local recurrence, 6) including reports about the percentage of vision-threatening complications, such as cataract and glaucoma following brachytherapy, 7) using 106Ru brachytherapy as a single primary treatment, 8) including more than 30 patients (eyes). Data were independently mined by three authors using a purpose-designed form. Following parameters were extracted from each study: number of patients, mean of tumor thickness (mm), follow-up time (months), radiation dose to tumor apex and sclera (Gy), local recurrence rate during follow-up (%), and rate of radiation-related cataract, glaucoma, papillopathy, and retinopathy (%). The present meta-analysis included the preferred reporting items for meta-analyses (PRISMA) checklist. Metaprop command Stata v.16 (StataCorp, Texas, USA) was used for data analysis. Estimates of the pooled proportion of local control and related confidence interval of 95% were combined using the inverse variance method. Between-study heterogeneity was assessed using Cochran Q and inconsistency index (I2). It was considered statistically significant when p-value was lower than 0.05, or I2 was higher than 50%. In case of a significant heterogeneity (I2 > 50%), a meta-regression analysis was used to evaluate the relationship between local control estimates and mean dose to the apex and mean tumor height.
Results
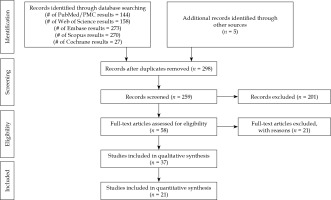
Twenty-one non-comparative observational studies were selected for this meta-analysis (Figure 1). Table 1 summarizes the studies included with their main descriptive characteristics [4-24]. Collectively, the studies involved 3,913 uveal melanomas with 106Ru brachytherapy plaques. The local control rate ranged from 59% to 98%, and the mean radiation dose to the apex of the tumor ranged from 70 Gy to 250 Gy.
Table 1
Descriptive characteristics of included studies
| Study [ref.] | Year | No. of patients | Mean tumor thickness (mm) | Mean dose to tumor apex (Gy) | Mean dose to sclera (Gy) | Mean follow-up (months) | Local control (%) | Post-treatment lens opacity (%) | Post-treatment glaucoma (%) | Retinopathy (%) | Papillopathy (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Jiang et al. [4] | 2020 | 39 | 3.70 | 141.4 | 557.7 | 69.5 | 87.1 | 53.8 | N.R. | 28.2 | 10.3 |
| Espensen et al. [5] | 2019 | 226 | 3.90 | 100.0 | N.R. | 60.0 | 78.0 | 45.5 | 12.0 | 28.3 | 27.4 |
| Rospond-Kubiak et al. [6] | 2017 | 126 | 4.80 | 100.0 | 570.0 | 66.5 | 86.5 | 5.0 | 4.0 | 39.6 | N.R. |
| Pagliara et al. [7] | 2017 | 239 | 3.29 | 99.99 | 268.4 | 48.0 | 91.6 | 4.2 | N.R. | 25.5 | 5.4 |
| Naseripour et al. [8] | 2016 | 51 | 8.12 | 71.0 | 1269.0 | 36.1 | 82.4 | 37.2 | N.R. | 53.0 | 29.4 |
| Fili et al. [9] | 2015 | 952 | – | 100.0 | – | 37.9 | 72.0 | N.R. | N.R. | N.R. | N.R. |
| Tarmann et al. [10] | 2015 | 143 | 4.50 | 89.8 | 689.8 | 37.88 | 88.2 | 40.6 | 10.5 | 16.8 | 25.2 |
| Salkola et al. [11] | 2014 | 45 | 1.90 | 116.0 | 327.0 | 62.0 | 91.0 | 28.0 | 2.0 | – | 2.0 |
| Takiar et al. [12] | 2013 | 40 | 3.05 | 90.0 | – | 67.0 | 97.0 | 50.0 | 5.0 | 50.0 | 2.5 |
| Perri et al. [13] | 2012 | 133 | 3.26 | 100.0 | 800.0 | 92.4 | 82.7 | 15.0 | 0.0 | 24.0 | 2.2 |
| Papageorgiou et al. [14] | 2010 | 189 | 3.70 | 105.5 | N.R. | 33.0 | 85.7 | N.R. | N.R. | N.R. | N.R. |
| Kaiserman et al. [15] | 2009 | 63 | 9.29 | 69.9 | N.R. | 69.6 | 76.2 | N.R. | 8.0 | N.R. | N.R. |
| Frenkel et al. [16] | 2009 | 413 | 4.70 | – | – | 66.6 | 86.0 | N.R. | N.R. | – | N.R. |
| Mossbok et al. [17] | 2007 | 45 | 5.33 | 113.7 | 821.5 | 61.6 | 84.0 | N.R. | N.R. | 20.0 | N.R. |
| Damato et al. [18] | 2005 | 458 | 3.20 | 115.0 | 400.0 | 46.8 | 98.0 | N.R. | N.R. | – | N.R. |
| Novak-Andrejcic et al. [19] | 2003 | 65 | 4.79 | 100.0 | N.R. | 90.8 | 84.6 | N.R. | N.R. | N.R. | N.R. |
| Georgopoulos et al. [20] | 2003 | 41 | 5.00 | 137.0 | 977.0 | 55.0 | 98.0 | N.R. | N.R. | N.R. | N.R. |
| Stoffelns et al. [21] | 2002 | 52 | 3.20 | 105.0 | N.R. | 67.2 | 92.0 | N.R. | 2.0 | 40.0 | 20.0 |
| Kleineidam et al. [22] | 1993 | 184 | 3.50 | 250.0 | 747.0 | 73.2 | 82.0 | N.R. | 0.5 | N.R. | N.R. |
| Summanen et al. [23] | 1993 | 100 | 6.00 | 100.0 | 1000.0 | 33.6 | 59.0 | 2.6 | 10.0 | N.R. | 10.0 |
| Lommatzsch et al. [24] | 1986 | 309 | N.R. | 100.0 | N.R. | 80.4 | 69.9 | N.R. | N.R. | N.R. | N.R. |
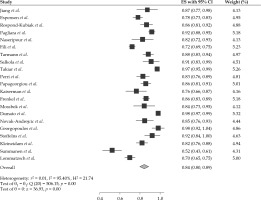
A random-effect model was used to analyze the efficacy of the treatment. The treatment’s overall efficacy in local control of the tumor was 84% (95% CI: 80-89%). The Cochrane Q analysis’s p-value was less than 0.05, emphasizing the heterogeneity of the studies’ results (Figure 2).
Fig. 2
Forest plot of 21 studies included in the meta-analysis to assess the efficacy of 106Ru brachytherapy in the treatment of uveal melanoma
As the I2 was 95.40%, the inconsistency between 106Ru efficacy reports was concluded. To explore the reason, meta-regression analysis was performed based on the mean of tumor height and the radiation dose to apex. Following meta-regression, the I2 index decreased to 92.52%, which showed that the inconsistency was not related to the heterogeneity between dose and tumor size (Figures 3, 4, and Table 2); however, the local control rate of the tumors was correlated with the mean tumor size (p-value = 0.024). More meta-regression and sub-group analysis were not applicable.
Fig. 3
The relationship between local control rates and mean tumor thickness. Circles were the surrogate for each study sample size
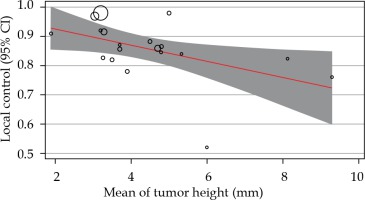
Fig. 4
The relationship between local control rates and mean radiation dose to the apex of the tumor. Circles were the surrogate for each study sample size
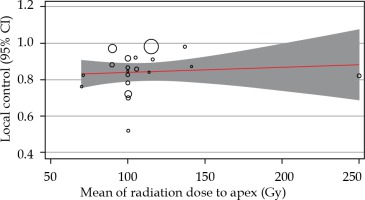
Table 2
The results of meta-regression based on tumor height and mean dose to the apex
The analysis of ocular complications was limited by authors’ poor reporting and was reflected in large and different results reported in the literature. According to the types of adverse effects, 14 studied described the rate of complications following brachytherapy [5, 6, 8-10, 14-16]. The rate of post-treatment retinopathy ranged from 20% to 53%. The rate of radiation-related crystalline lens opacity ranged from 4.2% to 53.8%. Radiation-related papillopathy and post-treatment ocular hypertension ranged from 2% to 29% and 2% to 12%, respectively.
Discussion
Our meta-analysis revealed a remarkable heterogeneity between the studies reporting the efficacy of 106Ru brachytherapy to treat uveal melanoma. However, 19 out of 21 reviewed studies reported a local control rate of more than 70%, and the weighted mean of this rate reached 84%.
Although the results are not conclusive, recent comparative studies have changed the primary concepts regarding the inferiority of 106Ru in local control of ocular melanoma. In a retrospective comparative case series with 2.5 years of follow-up, it has been reported that treatment with 106Ru is as effective as 125I [25]. Moreover, it has been suggested that 106Ru brachytherapy may be superior to 125I when the intended primary outcome is a reduction of thickness of melanoma. In another study comparing long-term efficacy and safety profiles of 106Ru and 125I brachytherapy, it was reported that both methods resulted in favorable control of the tumor, but 106Ru may provide additional benefit with reduced toxicity in tumors less than 5 mm of height [3].
The lower penetration power of 106Ru has limited its usage for melanomas thicker than five mm [3, 26]. Lack of reports on the effectiveness of 106Ru brachytherapy in treating large uveal melanoma makes it impossible to present a definite conclusion. In our study, 4 out of 21 reports have used 106Ru brachytherapy for tumors with a mean thickness of more than 5 mm, and reported rates of local control ranged from 59% to 84%. However, based on the negative slope of meta-regression, the thickness of uveal melanoma may be a predicting factor for the success of 106Ru brachytherapy, where the response of larger tumors could be lesser compared to smaller melanomas. Also, a minimal part of the heterogeneity in reported success rates was related to the tumors’ mean size.
Based on the previous reports and our analysis results, tumor location and radiation dose to the tumor’s apex seem to be additional determinants of therapeutic response to 106Ru plaque brachytherapy. It was suggested that the tumor location may be as important as the size of the treatment’s efficacy. In a study by Barker et al., tumors close to the edge of optic disc or the center of fovea, in addition to the cases with posterior tumor border near the posterior pole, were significantly associated with a higher rate of local recurrence. It may reflect the importance of tumor bulk coverage by the radiating plaque since, in the same study, smaller plaque diameter relative to the tumor’s largest base diameter was one of the predictors of 106Ru plaque brachytherapy failure [27].
In our meta-analysis, the correlation between the local control rates and the apex’s mean dose was positive but not statistically significant. Dose prescriptions for choroidal melanoma typically ranged from 70 Gy to 100 Gy to the tumor’s apex, with a treatment duration of 3 to 7 days. Although it may be interpreted as the presence of similar efficacy for different doses of apex radiation within the range of 70 Gy to more than 100 Gy, further studies should be conducted to evaluate this concept reliably. It would be logical to use lower doses to diminish the treatment’s short-term and long-term complications.
Predictably, the cumulative rate of local recurrence increases during the follow-up period. In a recent retrospective study, the local tumor recurrence rate increased from 3% at 12 months to nearly 15% at 48 months [10]. Similarly, uveal melanoma’s survival rates could decrease from 81.6% at five years to around 60% at ten years [10, 28]. After plaque brachytherapy, patients included in this review were followed for local control and complications with an interval of 3 to 6 months, and a follow-up duration of 1 to more than six years. The most common vision-threatening complications of 106Ru brachytherapy are retinopathy and cataract. However, ocular hypertension and optic neuropathy were also reported as complications of this treatment technique. According to the miscellaneous reports on the complications, plaque brachytherapy has also been associated with ocular surface disorders, sclera integrity, and ocular muscle functions [6, 10, 13, 22]. These complications must be treated through standard protocols to prevent loss of visual function and quality of life.
It seems that the preservation of visual acuity has been the primary goal of using beta-emitter isotopes in plaque brachytherapy. In preliminary non-comparative reports, assumed complications, such as neovascular glaucoma, are less common in patients treated with 106Ru [2]. According to previous reports, main risk factors associated with lower final visual acuity were older age, posterior and temporal tumor location, larger tumor, and posterior extension of the lesion [29]. Conservation of a visual acuity better than 20/200 and finger-counting were expected at long-term follow-up in 55% to 60% and over 80% of eyes treated with 106Ru, respectively [18]. Bergman et al. reported that up to 50% of patients treated with 106Ru were expected to have a visual acuity better than 0.1 in five years after the treatment [30].
Conclusions
The present study involves a systematic review of 21 retrospective studies. Our review’s main limitation was the retrospectivity of included studies, which made it impossible to perform a formal meta-analysis, since there were no randomized trials and prospective studies were rare. However, published studies suggest that plaque brachytherapy with 106Ru is successful in local control of uveal melanoma. Cataract and retinopathy are the main vision-threatening complication of radiation. Although pooled data analysis reveals a higher success rate in smaller tumors, this effect should be verified in randomized comparative studies.