Introduction
With the maturity of minimally invasive equipment and technology, laparoscopic minimally invasive surgery has been widely used in the diagnosis and treatment of gynaecological diseases due to its contributions to less trauma, mild postoperative pain, and rapid postoperative rehabilitation [1]. In clinical research, the selection of anaesthesia technique before surgery has steadily gained popularity. Currently, the most often used anaesthesia techniques in clinical practice are general anaesthesia with endotracheal intubation, epidural blocking anaesthesia, and combination spinal-epidural anaesthesia. However, different anaesthesia regimens can have varying impacts on body circulation and stress response, of which postoperative cognitive dysfunction (POCD) is the most common [2, 3]. POCD is defined as the presence of psychiatric symptoms such as memory loss, slurred speech, difficulty concentrating, poor numeracy, reduced logical ability, and delirium, as well as personality abnormalities and social movement disorders in patients after surgery. Also, it is one of the common surgical complications in clinics, especially in elderly patients receiving surgery under general anaesthesia [4, 5]. As the population ages and medical technology advances, more elderly gynaecological patients are opting for surgery as a treatment [6]. A current focus of clinical research is how to successfully prevent and cure POCD in elderly patients. Studies have shown that unstable transition during the recovery period after anaesthesia is one of the main factors leading to POCD in elderly patients. Aiming at this issue, maintaining haemodynamic stability and normal cerebral perfusion during the perioperative period in elderly patients is one of the main preventive measures [7].
Dexmedetomidine, a novel sedative agent, is a highly selective α2–adrenergic receptor agonist with central anti-sympathetic and anxiolytic effects [8]. Moreover, dexmedetomidine causes drowsiness that resembles a state of natural sleep in patients and modest respiratory depression [9]. Dexmedetomidine for sedative medication may have the ability to safeguard organs like the heart, brain, liver, and kidney [10]. Elderly gynaecological patients receiving laparoscopic surgery under general anaesthesia develop pain, poor tolerability of tracheal intubation completion, urinary catheter discomfort, and other issues before and after extubation in the recovery period. In this case, the patients will be quite uncomfortable and prone to agitation [11]. Also, the severe fluctuations it causes in heart rate (HR), blood pressure, and other vital signs increase the risk of postoperative POCD [12]. Therefore, the patients in this study were given a continuous load injection of dexmedetomidine before the end of surgery. This was done to ensure the patients’ haemodynamic stability and to help them get through the post-operative period more smoothly, as well as to reduce the stress response brought on by extubation during the recovery period.
Aim
This study wished to take this opportunity to outline the pertinent clinical evidence supporting the application of dexmedetomidine in elderly gynaecological patients undergoing laparoscopic surgery.
Material and methods
Study subjects
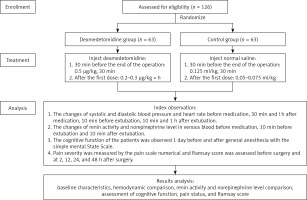
A total of 126 elderly patients who had underwent elective gynaecological surgery under general anaesthesia in our hospital from March 2017 to March 2019 were divided into a dexmedetomidine group (n = 63) and a control group (n = 63) using a random number table. General data of patients such as age, American Society of Anesthesiologists (ASA) physical status, operation duration, and intraoperative blood loss were collected. Inclusion criteria and exclusion criteria [13] of the participants were as follows. Inclusion criteria: (1) elderly gynaecological patients aged 60 to 80 years; and (2) patients who had received elective gynaecological laparoscopic surgery under general anaesthesia. Exclusion criteria: patients with (1) long-term medication of benzodiazepines; (2) severe liver, kidney, and lung dysfunction; (3) a history of mental or neurological diseases; (4) a history of alcoholism and drug abuse; (5) serious adverse clinical events during surgery such as shock and cardiac arrest; (6) sinus bradycardia; and (7) successful postoperative recovery and the time from the end of surgery to extubation < 30 min. All patients and their families who were informed of the study objective and content signed the informed consent forms. This study has been approved by the Ethics Committee of Shijiazhuang Sixth Hospital (Ethics No. 202012) (Figure 1).
Anaesthesia and treatment
All patients were given endotracheal intubation under general anaesthesia. Peripheral venous access was established, HR was monitored using a multifunctional monitor, and diastolic blood pressure and systolic blood pressure changes were recorded using a noninvasive blood pressure meter. All patients were given propofol (1 mg/kg), sufentanil (0.5 μg/kg), and atracurium (0.15 mg/kg) for anaesthesia induction. After successful intubation, respiration was controlled by an anaesthesia machine with a tidal volume (VT) of 6–12 ml/kg, respiratory rate (RR) of 12 breaths/min, and inspiratory and expiratory ratio of 1 : 2. Subsequently, the patients received continuous intravenous infusion of propofol (75 μg/kg·min) and remifentanil (0.3 μg/kg·min) with a micropump and intermittent intravenous injection of cisatracurium (0.03 mg/kg) for anaesthesia maintenance.
Patients in the dexmedetomidine group were treated with dexmedetomidine (Jiangsu Hengrui Pharmaceuticals Co., Ltd.; specification; 2 ml/200 μg; approval number: 10122134) 30 min before the end of surgery. 2 ml dexmetomidine was added to 48 ml normal saline to obtain a solution with a total volume of 50 ml. At first, 0.5 μg/kg of dexmedetomidine was pumped for sedation induction for 30 min. Later, a micropump was used for continuous infusion of dexmedetomidine (0.2–0.3 μg/kg·h). Patients in the control group were given the same amount of saline infusion during the corresponding time.
Outcome measures
The changes of systolic blood pressure (SBP), diastolic blood pressure (DBP), and HR were observed before medication (T0), 30 min after medication (T1, at the end of surgery), 1 h after medication (T2, 0.5 h after the end of surgery), 10 min before extubation (T3), 10 min after extubation (T4), and 1 h after extubation (T5), in both groups.
The changes in renin activity and norepinephrine level at T0, T3, and T4 were observed. Five millilitres of venous blood were drawn from all included patients at the above time points and fully mixed in heparinized centrifuge tubes. The centrifugation speed was set at 3000 r/min and the centrifugation radius was 12 cm. Fifteen minutes later, serum was separated and stored in a freezer at –20°C. Next, 3 ml of the serum was taken to determine renin and norepinephrine levels. Specifically, the noradrenaline level was detected by the HP1050 high-performance liquid chromatography system, an HP1049 electrochemical detector, with the help of the ion-pair reversed-phase chromatography-electrochemical method. Renin activity was detected by radioimmunoassay. The kit was provided by Beijing North Institute of Biotechnology Co. Ltd. The specific operation was performed according to the operating process of the kit.
Mini-mental state examination (MMSE) was used to assess the cognitive function of the patients 1 day before surgery and 1 day after surgery. A visual analogue scale (VAS) was used to evaluate the pain before surgery, and 2, 4, 12, 24, and 48 h after surgery. The Ramsay score was applied to assess the state of consciousness before surgery, and 4, 12, 24, and 48 h after surgery [14].
Statistical analysis
SPSS 19.0 statistical software was used for data analysis. Measurement data were expressed as mean ± standard deviation (SD), and the t-test was employed for comparison between groups; enumeration data were expressed as n, and the χ2 test was performed for comparison between groups. P < 0.05 was considered to indicate a statistically significant difference.
Results
General clinical data of patients in the 2 groups
As shown in Table I, a total of 126 patients were included in this study. There was no significant difference in age, body mass index (BMI), childbearing history, ASA physical status, operation duration, and intraoperative blood loss between the 2 groups (p > 0.05). The results suggest that the general data of the 2 groups were comparable.
Table I
Comparison of general clinical data of patients
Comparison of haemodynamics between the 2 groups
As shown in Table II, there were no significant differences in SBP, DBP, and HR between the 2 groups before treatment (T0), and 30 min (T1) and 1 h after medication (T2) (p > 0.05). After treatment, SBP and DBP decreased to the normal range in both groups. At T3, T4, and T5 there were significant differences in SBP, DBP, and HR between the 2 groups (p < 0.05).
Table II
Comparison of haemodynamics between the 2 groups
[i] T0 – before medication, T1 – 30 min after medication (at the end of surgery), T2 – 1 h after medication (0.5 h after the end of surgery), T3 – 10 min before extubation, T4 – 10 min after extubation, T5 – 1 h after extubation, SBP – systolic blood pressure, DBP – diastolic blood pressure. Data expressed as mean ± SD. The t-test was used to compare the differences between the 2 groups.
Comparison of renin activity and norepinephrine level between the 2 groups
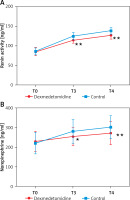
The results showed that there were no significant differences in renin activity and norepinephrine level before treatment between the 2 groups (p > 0.05). At T3 and T4, renin activity and norepinephrine level were significantly increased in both groups, but the dexmedetomidine group showed much lower renin activity and norepinephrine level than the control group (p < 0.05) (Figure 2).
Figure 2
Comparison of renin activity and norepinephrine level between the 2 groups. A – Comparison of renin activity between the 2 groups; B – Comparison of norepinephrine level between the 2 groups
T0 – before medication, T3 – 10 min before extubation, T4 – 10 min after extubation; *P < 0.05 and **P < 0.01 vs. the control group.
Cognitive function assessment in the 2 groups
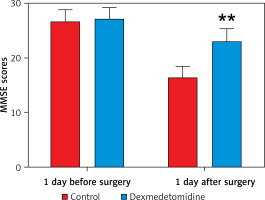
No significant difference existed in MMSE score 1 day before surgery between the 2 groups (p > 0.05). However, the MMSE score in the dexmedetomidine group 1 day after surgery was much higher than that in the control group, and the difference was statistically significant (p < 0.01) (Figure 3).
Comparison of pain level and Ramsay score between the 2 groups
As shown in Table III, there were no significant differences in VAS and Ramsay scores between the 2 groups before surgery (p > 0.05). The VAS score in the dexmedetomidine group was observably lower than that in the control group at 2, 4, 12, 24, and 48 h after surgery (p < 0.001). At 12 h after surgery, the Ramsay score in the dexmedetomidine group was significantly higher than that in the control group (p < 0.001). Notably, there was no significant difference in the Ramsay score between the two groups at 24 and 48 h after surgery (p > 0.05).
Table III
Comparison of pain level and Ramsay score between the 2 groups
Discussion
Elderly gynaecological patients after laparoscopic surgery under general anaesthesia have poor tolerance to surgery, and their cerebral circulation function is easily damaged due to factors such as the decreased mutual coordination of body organs, a greater impact of CO2 pneumoperitoneum on respiratory circulation, and the particularity of intraoperative position; the patients are also susceptible to postoperative pain, anxiety, excessive stress, and emergence agitation, as well as complications such as POCD [15]. Relevant studies have shown that there is a linear relationship between POCD and patient age; more specifically, the incidence of POCD increases significantly with age. The type of surgery, operation duration, trauma size, anaesthetic drugs, and the degree of previous organ reserve function of patients are risk factors for POCD [16, 17]. Exploring the effective prevention and treatment of POCD in elderly patients undergoing surgery has emerged as one of the major areas of clinical medical study as a result of the aggravation of population aging in China and the rise in the number of elderly patients receiving surgical care [18]. To avoid postoperative problems and promote postoperative rehabilitation, it is crucial to choose a scientific anaesthesia regimen for elderly patients receiving general anaesthesia for surgery.
Recent years have seen very positive results from combination anaesthesia in laparoscopic surgery due to advancements in science and technology [19]. Unfortunately, this method has certain limitations, such as its inability to precisely determine the pharmacokinetic and pharmacokinetic characteristics of a single type of anaesthetics. Dexmedetomidine, a highly potent novel α2-adrenoceptor agonist [20], has been reported to cause modest respiratory depression, play a calming role that is similar to that of natural sleep, and exert a good antagonistic effect on central sympathetic activation. Hence, it is widely used in clinical practice for sedation during endotracheal intubation and mechanical ventilation in patients under general anaesthesia [17, 21]. Some scholars have reported that dexmedetomidine can help patients get through the perioperative phase without impairing breathing, and it may preserve the function of the heart, brain, liver, kidney, and other organs during sedative treatment [22]. With the aforementioned benefits of dexmedetomidine in mind, this study investigated the endocrine hormone activity and haemodynamic characteristics of elderly gynaecological patients receiving laparoscopic surgery under general anaesthesia before and after dexmedetomidine administration. It also looked at the clinical effect of dexmedetomidine based on MMSE, VAS, and Ramsay scores.
In this study, the clinical baseline characteristics showed no significant differences between the 2 groups and were comparable. The results of haemodynamic tests showed that SBP, DBP, and HR were much lower in the dexmedetomidine group than those in the control group at T3 and T4, suggesting that dexmedetomidine can maintain stable haemodynamic parameters during emergence from anaesthesia in elderly patients [23]. The capacity of dexmedetomidine to lower HR and blood pressure as well as maintain steady blood circulation by reducing sympathetic excitability is a plausible explanation for this. Additionally, the levels of endocrine hormones in the 2 groups were compared. Our findings revealed that at T3 and T4 the dexmedetomidine group displayed a significantly lower level of renin activity and norepinephrine than the control group, indicating that the use of the drug is advantageous for maintaining stable hormone levels during extubation. Key findings in the study of Asad included the same demonstration [24]. MMSE, VAS, and Ramsay scores were evaluated after operation, and the results showed that VAS scores in dexmedetomidine group were obviously lower than those in the control group, while MMSE and the Ramsay score in the dexmedetomidine group were apparently higher than that in control group at 12 h after operation. The outcomes demonstrated that dexmedetomidine could reduce the likelihood of postoperative cognitive dysfunction, enhance cognitive function, and relieve pain in elderly patients. In multiple meta-analyses, dexmedetomidine significantly reduced the risk of early POCD during the perioperative period and improved postoperative MMSE score in elderly patients, which is consistent with our findings [25, 26].
Dexmedetomidine is a potent, highly selective agonist of pre- and post-synaptic α2-adrenoceptors in the central nervous system [20]. In addition to the pre-sympathetic junction, α2-adrenoceptors are also expressed on vascular smooth muscle cells [27]. The mechanism of action of dexmedetomidine has been described as follows: dexmedetomidine weakens sympathetic excitability by binding to α2-adrenoceptors, which inhibits excitatory neurotransmitter release; at the same time, cholinergic anti-inflammatory pathways are activated to reduce inflammatory factor release as well as the inflammatory response; accordingly, haemodynamic parameters are stabilized and the risk of postoperative cognitive dysfunction is lowered [28]. In the study of Wang et al., dexmedetomidine was found to increase miR-381 expression and to inhibit early growth response protein 1 (EGR1)-mediated p53 activation, thereby reducing DNA damage, neuroinflammatory response, neuronal apoptosis, and postoperative cognitive dysfunction in elderly patients [29]. In a meta-analysis, the investigators confirmed that dexmedetomidine significantly reduced opioid consumption by patients for 24 h after laparoscopy and prolonged the time to first request analgesia [30]. Dexmedetomidine has also been demonstrated to block the function of the central nervous system, lowering noradrenergic and dopaminergic output in a dose-dependent manner [31]. Dexmedetomidine may enhance postsynaptic dorsal root ganglion-mediated analgesia by inhibiting central noradrenergic pathways [32]. In our study, dexmedetomidine was beneficial to maintain stable hormone levels during extubation and stable haemodynamic parameters during anaesthesia recovery, as well as to improve cognitive function and pain in elderly gynaecological patients. It is obvious that our findings are basically consistent with previous studies [33].
However, there are some limitations to this study. For example, the sample size of this study is small. Future studies with multiple centres and large sample sizes will be required to validate the findings of this study.
Conclusions
Taken as a whole, dexmedetomidine can stabilize the haemodynamic parameters and the hormone levels, effectively relieve postoperative pain, and enhance the postoperative cognitive function in elderly gynaecological patients undergoing laparoscopic surgery during anaesthesia recovery. It is deserving of widespread public awareness due to its superior overall effect and strong clinical applicability.