Introduction
The incidence of congenital hypothyroidism in Indian babies is reported to be 1 in 600 (Eastern India); 1 in 727 (Southern India); 1 in 3400 (Chandigarh); 1 in 1140 (Lucknow) from North India; and 1 in 1700 (Hyderabad), which is much higher than the reported incidence from western literature of 1 in 3500 [1–6]. There are few data on congenital hypothyroidism in preterm babies. The physiological immaturity of the axis, the low maternal contribution, illnesses, and drugs have variable influences on the thyroid functional status [7, 8]. The pivotal question, whether the prevalence of congenital hypothyroidism is higher in preterms, is debatable. Extensive previous research reveals both comparable [9, 10] and higher prevalence [11–13] compared to term babies. The variable results may be due to the different study populations and varied cut-offs used in various studies.
Conventionally, preterm babies are suspected to be more prone to transient congenital hypothyroidism because of perinatal factors [14]. However, recent research has identified a significant presence of permanent hypothyroidism in preterm babies [15]. This has pertinent implications for the counselling of the families at the time of initiation of thyroxine therapy in the neonatal period.
Screening for congenital hypothyroidism in preterm babies is a classic case of double whammy. On the one hand there is a risk of missing cases of congenital hypothyroidism owing to immaturity of the hypothalamo-pituitary thyroid axis. On the other hand there is a risk of overdiagnosing cases of congenital hypothyroidism due to physiological alterations in the HPT axis [16]. The interpretation of thyroid function test in a preterm baby must be done carefully.
The need for a second thyroid function test in these babies is debatable. Several studies have supported the need for a venous sample at term or 36 weeks post menstrual age to increase the sensitivity of pick-up [11, 12, 15–17]. There are also studies that quote no added value of a repeat test as long as the initial cut-off of TSH is low [13]. There are no Indian data in this regard.
With this background, we performed this study to ascertain the prevalence of congenital hypothyroidism in preterm babies in our unit, present their aetiological profile and ascertain the utility of a repeat venous testing in preterm babies.
Material and methods
We conducted a prospective observational study over a period of 3 years (2015–2018).
From amongst all babies either delivered at, or referred to, a tertiary care centre in South India, we included babies born < 37 weeks of gestation. We excluded babies with Down syndrome, death, or against medical advice, those who were transfused prior to sampling, and those who could not be screened for congenital hypothyroidism. Sample was collected at 72–120 hours of life, irrespective of gestational age, by heel prick by a single trained technician in pre-marked circles of 1 cm diameter on Schleiecher and Schuell specimen collection filter paper. The filter papers were air dried and transported, and TSH levels were estimated by Sandwich Enzyme Linked Immunoassay using Bio-rad Quantase TM kit (lower detection limit: 0.05 µIU/mL and upper detection limit: 100 µIU/ml).
The gestational age, sex, and heel prick TSH were collected prospectively. Data pertaining to maternal (age, hypertension, diabetes mellitus, hypothyroidism), perinatal factors (mode of delivery), and neonatal factors (birth weight, sex, gestational age, presence of neonatal complications) were collected and entered.
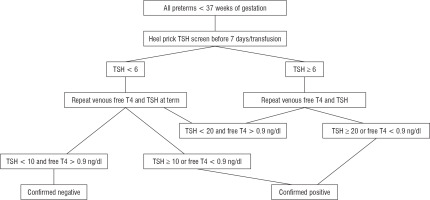
The study protocol is described in Fig. 1 [18, 19]. All screen-positive cases underwent venous testing immediately. We considered heel prick TSH ≥ 6 µIU/ml as screen positive; those with venous TSH ≥ 20 or free T4 < 0.9 ng/dl at GA < 37 weeks or venous TSH ≥ 10 or Free T4 < 0.9 ng/dl in a term infant as confirmed positive. However, those with screen positive and venous TSH < 20 and Free T4 > 0.9 ng/dl were followed up and retested by a venous sample for Free T4 and TSH at term. All screen-negative cases also underwent venous testing at term. Babies with congenital hypothyroidism were evaluated and with Anti-thyroid peroxidase antibody, anti-thyroglobulin antibody, ultrasound of the thyroid, and technetium thyroid scintigraphy, where feasible. They were initiated on thyroxine therapy in a dose of 10–15 µg/kg/day. Subsequently, these babies were followed up in the thyroid clinic every 2 weeks until TSH normalized, twice monthly in the first year of life and thrice monthly thereafter [18, 19]. Study was approved by the Institute Ethics Committee. Collected data were entered in Microsoft Excel and analysed. All data were presented as mean ± SD. Pearson correlation was used to determine the correlation between screening and venous TSH.
Results
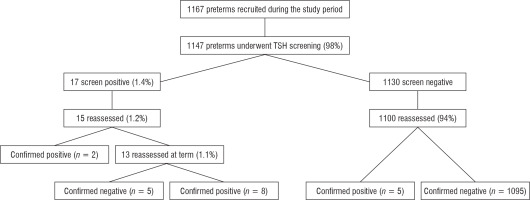
A total of 1167 preterm babies presented at our unit during the study period, of whom 1147 (98%) underwent TSH screening. The mean ± SD of TSH at different gestational ages are described in Table 1. Of the 1147 preterm babies, 17 (1.4%) were screen positive. A comparison of the maternal (age, hypertension, diabetes mellitus, hypothyroidism), perinatal (mode of delivery), and neonatal factors (birth weight, sex, gestational age, presence of neonatal complications) between the preterm babies with and without positive screening for congenital hypothyroidism is depicted in Table 2.
Table I
Screening thyroid stimulating hormone (TSH) at different gestational ages in the study population
| Gestational age (in weeks) | TSH values (in µIU/ml) |
|---|---|
| 28 weeks | 2.0 ±0.5 |
| 29 weeks | 1.9 ±0.7 |
| 30 weeks | 2.0 ±0.6 |
| 31 weeks | 2.1 ±0.8 |
| 32 weeks | 2.1 ±0.8 |
| 33 weeks | 1.7 ±0.7 |
| 34 weeks | 1.8 ±0.7 |
| 35 weeks | 2.0 ±0.5 |
| 36 weeks | 2.0 ±0.4 |
Table II
Comparison of maternal, perinatal, and neonatal factors in preterm babies who were screen positive and screen negative
Out of these 17 babies who were screen positive, 15 underwent confirmatory venous test: Two were confirmed to have congenital hypothyroidism as preterm babies. The remaining 13 babies underwent retest venous sample at term, and 8 of these were confirmed to have and 5 not to have congenital hypothyroidism. Of the screen-negative babies, 1100 underwent repeat venous testing at term/ prior to discharge. Five out of these 1100 babies were confirmed to have congenital hypothyroidism. Thus, 15 babies out of 1147 screened babies were found to have congenital hypothyroidism (Fig. 2) leading to a prevalence of 1 in 77 screened babies. We attempted to study the correlation between neonatal TSH screen and venous TSH in the study population. No correlation was observed between screening TSH and venous TSH (p > 0.05).
None of the preterm babies with congenital hypothyroidism had clinical signs or symptoms of congenital hypothyroidism. Out of the 15 cases of congenital hypothyroidism, evaluation was feasible in 8 cases. Aetiological evaluation revealed transient causes secondary to maternal antibody in 4 cases (50%) and permanent thyroid defects in 4 cases (50%). Permanent thyroid defects observed in the study include dyshormonogenesis (n = 2), agenesis (n = 1), and ectopic lingual thyroid (n = 1).
All babies with confirmed hypothyroidism were initiated on thyroxine replacement in a dose of 10–15 µg/kg (mean dose 11.5 ±1.1 µg/kg/day) and followed up in the thyroid clinic.
Discussion
To the best of the author’s knowledge, this is the first Indian study on congenital hypothyroidism in preterm babies. We have described the experience of our unit in screening and evaluating preterm babies with congenital hypothyroidism. In the present study, we have observed a prevalence of 1 in 77 among the 1147 preterm babies screened. Five (33%) out of these cases were missed on initial screening, reiterating the need for repeat testing, and 5 of the screen-positive cases did not have the disease, reiterating the need to follow the standard protocols in preterm babies
In our study, we have 15 cases of confirmed congenital hypothyroidism out of 1147 screened preterms, resulting in a prevalence of 1 in 77. This prevalence is much higher than term babies. Our results are comparable to reports by Silva et al. [20], Tylek et al. [11], Bijamia et al. [12], and Korada et al. [13], who reported a prevalence of 1 in 242, 1 in 202, 1 in 128, and 1 in 560, respectively. However, Srinivasan et al. [9] and Mao et al. [10] did not report a similar increase. This may be attributable to the fact that these tests have been done on healthy babies, but ours is a tertiary referral unit where many high-risk babies are seen. The influence of perinatal risk factors on increased prevalence of CH has been described previously [21]. Also, a significant number of our preterms underwent interventions like surfactant administration, enterocolitis, and ductus closure in the first week of life (Table II). However, the high prevalence in preterm babies in our study is an indication to screen all preterm babies for congenital hypothyroidism.
In our study, we evaluated 8 out of the 15 confirmed cases; 50% each were transient and permanent aetiologies. The other studies have quoted 60%, 45.4%, and 40% permanent aetiologies for congenital hypothyroidism in preterm babies [9, 11, 15]. The variability may be attributable to the different definitions adopted in various studies for permanency: Srinivasan et al. [9] defined requirement of thyroxine beyond 3 years as permanent. We have adopted universally acceptable scintigraphic evidence to determine permanent congenital hypothyroidism. Notwithstanding, the high prevalence of permanent congenital hypothyroidism suggests that aetiological evaluation is mandatory in these high-risk babies, either at birth or at 3 years of age.
Five cases of congenital hypothyroidism had normal initial TSH screen. These cases would have been missed if the TSH screening was not repeated at term. Similarly, Silva et al. [20], Bijamia et al. [12], and Vigone et al. [15] reported that 90.9%, 88.8%, and 73.9% of the cases would have been missed if the initial screening were not repeated. This may be attributable to the neonatal illnesses in a preterm baby, which may lead to suppression of the HPT axis. Considering the importance of early therapy for congenital hypothyroidism, it is essential that all preterms are retested at term or prior to discharge, irrespective of the initial thyroid screening results.
The Indian Society of Paediatric and Adolescent Endocrinology (ISPAE) have given guidelines for management of congenital hypothyroidism [22, 23]. However, we used the guidelines recommended by the ESPE [19] because these babies were managed prior to the publishing of the ISPAE guidelines. We attempted to reanalyse our data using the ISPAE guidelines: 7 babies were screen negative, as per the ISPAE guidelines, and screen positive as per the ESPE guidelines. Of these 7 babies, 3 were found to have congenital hypothyroidism at term. None of the babies with congenital hypothyroidism identified as preterm would have been missed as per either guidelines. Thus, the new ISPAE guidelines may be more cost effective in developing countries like ours.
Our study is not without limitations. Aetiological profiling was possible in 8 out of 15 cases due to logistic issues. However, the remaining 7 babies are being followed up in the thyroid clinic. We are planning a trial off therapy at 3 years of age and aetiological assessment in these infants at 3 years of age. Also, the small sample size in our study is a significant limitation.
To summarise, we have described the experience of our unit in screening and evaluating preterm babies for congenital hypothyroidism. We observed a high prevalence (1 in 77). A need for repeat venous testing, irrespective of initial screening, and significant permanent congenital hypothyroidism (50%) were observed in our series.

 POLSKI
POLSKI