Purpose
In 2020, the global incidence of colorectal cancer ranked third highest of all cancer types, with 10%. Furthermore, colorectal cancer accounted for 9.4% of all cancer-related deaths, considering all sexes and age groups [1]. Up to 50% of all colorectal cancer patients develop colorectal liver metastases (CRLM) over the course of the disease, mostly due to hematogenic seeding [2]. In patients with unresectable liver metastases or patients with health conditions that do not allow surgical procedures, minimally invasive, locoregional procedures may be considered [3]. The decision for or against the resection of CRLM is sometimes challenging and based on an array of variables, e.g., size, localization, nodularity, and further underlying diseases [4]. Moreover, the extent of excised liver parenchyma, number of metastases, diameter of the largest lesion, extension of the primary tumor into the serosa as well as lymphatic spread and patient’s age, show significant impact on long-term survival in case of surgical resection [5]. 5-year survival rates in patients with CRLM after surgical resection range from 25% to 59% [6-8].
Local ablative methods, such as computed tomography-guided high-dose-rate brachytherapy (CT-HDRBT) have proven effective in treating primary and secondary liver tumors. In CRLM, studies showed median overall survival (OS) times ranging from 14 months to 23.4 months, depending on the applied target dose and size of liver metastases. Although there is no defined size limitation of lesions suitable for CT-HDRBT, it is recommended for lesions with a maximum diameter of 4 to 5 cm and, if possible, the treatment should be combined with other local or systemic therapies to improve outcomes [9, 10].
Transarterial chemoembolization (TACE), e.g., with the topoisomerase-I-inhibitor irinotecan, is a widely used locoregional therapy for unresectable CRLM. Median OS times ranging from 8 months to 18.2 months were reported for patients with CRLM treated with irinotecan-TACE [11-13]. Both methods, i.e., CT-HDRBT and irinotecan-TACE, are safe and well-tolerated, with no or hardly any major or minor complications, even in a combined setting [14, 15].
The combination of CT-HDRBT and irinotecan-TACE in treating CRLM is scarcely studied and has not yet been compared in terms of efficacy and toxicity with CT-HDRBT alone. To support treatment recommendations in the palliative management of CRLM, this study presents a first-time assessment of local tumor control (LTC), progression-free survival (PFS), and toxicity profiles of mono-CT-HDRBT compared with a combination therapy of irinotecan-TACE and CT-HDRBT in matched patients with large, unresectable CRLM.
Material and methods
Study design
Ethics approval was granted by the institutional review board (EA1/043/15) in accordance with the Declaration of Helsinki. The first cohort (n = 23) was part of a prospective study receiving combination therapy of irinotecan-TACE and CT-HDRBT for large CRLM with a diameter of > 3 cm between 2015 and 2017 [14]. For the second cohort, patients treated with mono-CT-HDRBT were retrospectively matched to the first cohort’s patients.
Inclusion criteria were a primary colorectal tumor with hepatic metastases classified as non-R0-resectable, patients with n ≤ 3 liver metastases with a diameter of > 3 cm, and an estimated life expectancy of more than 3 months. Exclusion criteria consisted of portal vein thrombosis diagnosed by CT, magnetic resonance imaging (MRI), or sonography, previous treatments with selective internal radioembolization, TACE, or CT-HDRBT for the same target lesions, and uncontrolled extrahepatic disease.
Matching process
The matching was performed in two subsequent stages. Since our study investigated the efficacy and toxicity of locoregional therapy in large CRLM with either combination of irinotecan-TACE and CT-HDRBT or mono-CT-HDRBT, a pre-selection of potential matching partners with monotherapy from our database was performed for each combination therapy patient by accounting for factors that can particularly influence both endpoints. These included: clinical target volume (CTV) of ±10%, lesion count of ±1, selected target volume isodose of either 15 or 20 Gy, number of catheters used of ±1, and CTV coverage with the chosen isodose of ±10% for the treatment with CT-HDRBT performed in both groups, as well as pre- and post-interventional treatment characteristics (resection, systemic, locoregional) and the presence of extrahepatic disease. After identifying all patients with mono-CT-HDRBT who matched patients from the combination therapy cohort for these criteria, the remaining mono-CT-HDRBT candidates were considered for propensity score matching using the nearest neighbor method without replacement, adjusting for the above characteristics as well as age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, tumor distribution, and synchronous/metachronous metastases (Tables 1 and 2). Molecular tumor genetics could not be retrospectively considered for matching due to partially missing data. Finally, one mono-CT-HDRBT patient with the smallest propensity score difference was selected as a matching partner for each patient who received combination therapy of CT-HDRBT and irinotecan-TACE.
Table 1
Patient baseline and disease-specific characteristics of the cohorts with combined CT-guided high-dose-rate brachytherapy (CT-HDRBT) and transarterial chemoembolization with irinotecan (irinotecan-TACE) or mono-CT-HDRBT treatment
Table 2
Treatment characteristics of combination therapy with CT-guided high-dose-rate brachytherapy (CT-HDRBT) and transarterial chemoembolization with irinotecan (irinotecan-TACE) or mono-CT-HDRBT treatment and post-procedural therapies
Peri-interventional workup
Initial imaging was performed with a 1.5 Tesla contrast-enhanced MRI of the liver with Gd-EOB-DTPA (e.g., Primovist®, Bayer Vital, Leverkusen, Germany; Figure 1) and a CT of the thorax to control for new extrahepatic metastases or consolidations. Laboratory workups were performed on the day before and after each intervention to assess blood count toxicity. Immediate follow-up examinations occurred the day after each intervention, including sonography of the liver or the punctured femoral artery. Post-interventional control imaging was scheduled in 3-month intervals using contrast-enhanced CT or MRI of the abdomen.
Fig. 1
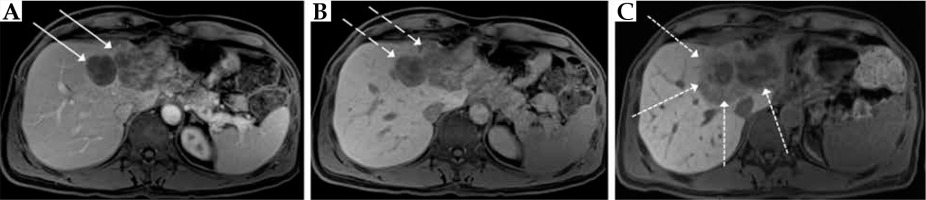
Pre- (A, B) and 60-days post-interventional MRI (C) in a patient with colorectal liver metastases in the left liver lobe after treatment with irinotecan transarterial chemoembolization and CT-guided high-dose-rate brachytherapy. A) Fat-saturated T1 transverse sequence after Gd-EOB-DTPA-injection in the portal venous phase with an inhomogeneous enhancement of the liver metastases in liver segments II and IVa (arrows); B) Fat-saturated T1 transverse sequence with missing Gd-EOB-DTPA-retention in the liver metastases (dashed arrows); C) Fat-saturated T1 transverse sequence with missing Gd-EOB-DTPA-retention in the liver metastases as well as perifocally corresponding to irradiation volume (dotted arrows)
Irinotecan-TACE
Patients receiving combination therapy had irinotecan-TACE as the first treatment 1 to 4 days before CT-HDRBT. Interventional access was granted via the femoral artery with the insertion of a 5-F sheath in Seldinger’s technique. Orientating coeliacography and mesentericography were acquired. After mixing 50 mg microspheres (100 µm in diameter; Embozene TANDEM™; Boston Scientific, Malborough, Massachusetts, USA) per ml saline solution with 10 ml contrast agent, the application was performed through a 2.5-F microcatheter into the tumor-supplying segmental arteries until stasis of the blood flow or the pre-described maximum dose of up to 150 mg microspheres was reached (Figure 2).
Fig. 2
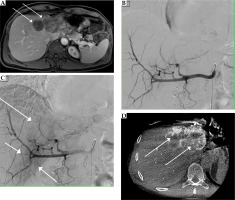
The same patient as in Figure 1, with confluent colorectal liver metastases in the left liver lobe. A) Pre-interventional MRI shows inhomogeneous Gd-EOB-DTPA enhancement of the metastases (arrows) in the left liver lobe (dynamic T1 axial in portal venous phase). Coeliacography with contrast-enhanced common hepatic artery early (B) after injection. Later (C), there is a very faint blush in the region of the left liver lobe (arrows), which corresponds to the metastases. Cone-beam-CT (D) was performed from the common hepatic artery with visualization of the hypovascularized target tumor in the liver segments II and IVa (arrows) for transarterial chemoembolization planning
CT-HDRBT
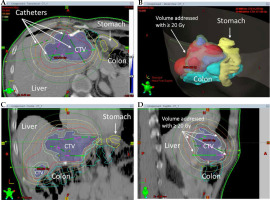
The positions of the respective target lesions were re-evaluated with a native spiral CT before the intervention. Puncturing was performed with a 17-G needle under local anesthesia, placing a flexible 230 mm long 6-F catheter sheath (Cordis AVANTI™ + Sheath Introducer, 0.035”; Miami Lakes, Florida, USA) over a stiff angiographic guidewire (Amplatz Super Stiff™, 145 cm, 0.035”, Boston Scientific; Boston, Massachusetts, USA) using Seldinger’s technique. Finally, the 350 mm 6-F afterloading catheter (Primed® Halberstadt Medizintechnik GmbH, Halberstadt, Germany) was placed, and the sheath was fixed to the patient’s skin with sutures. 3D irradiation planning for brachytherapy was performed by a medical physics expert and a specially trained interventional radiologist using Brachyvision™ software (Gammamed™, Varian; Palo Alto, California, USA; Figure 3). Catheters, CTV, and potential risk structures were registered manually, followed by semi-automatic calculation of brachytherapy plan consisting of the iridium-192 (192Ir) source’s age, dwell times, and respective locations inside the catheters to cover the CTV with the intended isodose. CTV referred to the liver tissue volume containing gross tumor volume and microscopic tumor environment up to 2 cm from the tumor margin, considering the internal organ movement. The desired CTV enclosing dose was 20 Gy but was reduced to 15 Gy in patients where adjacent structures were at risk (e.g., stomach and small intestine). After planning the 192Ir radiation source was remotely inserted into the afterloading catheter. Irradiation time varied among the patients, depending on the semi-automatic calculation to achieve full planned coverage of the CTV with 20 or 15 Gy.
Study endpoints
Retrospective assessment of tumor progress/evaluation of extrahepatic disease was performed by two board-certified radiologists with five and seven years of experience in body radiology, respectively (SF, MJ). Local recurrence was assessed according to the new response evaluation criteria in solid tumors (version 1.1) [16]. Progression-free survival corresponded to the time without local, intra-, and extrahepatic progress. Due to retrospective study design and partially missing data of the patients’ survival status, overall survival (OS) could not be analyzed.
Changes in the blood parameters, including total bilirubin, alanine aminotransferase (ALT), alkaline phosphatase (AP), activated partial thromboplastin time (aPTT), aspartate aminotransferase (AST), γ-glutamyltransferase (GGT), international normalized ratio (INR), and hemoglobin were documented using classification into toxicity levels via National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0) one day before and after treatment [17]. Matched pairs with missing values were excluded from each sub-analysis. Moreover, catheter-related adverse events were documented and graded according to the Society of Interventional Radiology classification [18].
Statistical analysis
Microsoft Excel 2016 (Microsoft®; Redmond, Washington, USA) and SPSS Statistics 25 (IBM®; Armonk, New York, USA) were used for statistical analysis. Categorical variables were reported as frequencies/percentages and continuous data using mean/standard deviation or median/interquartile range. Normal distribution was examined using the Shapiro-Wilk test. Cohort characteristics after matching were compared using the paired sample t-test, Wilcoxon, and McNemar test. Statistical estimation of LTC and PFS was performed using the Kaplan-Meier estimator and log-rank test. Risk factors of poorer LTC or PFS were analyzed with univariable/multivariable Cox regression. Variables with a p-value of < 0.1 in univariable analysis were included for multivariable backward Cox regression. Multicollinearity was tested with Spearman’s correlation analysis. Receiver operating characteristic (ROC) curve analysis with Youden’s index was applied to identify cut-off values for significant variables. P-values < 0.05 were considered significant.
Results
Matching results
A total of 423 patients with mono-CT-HDRBT treatment were identified in our internal database between 2008 and 2018. Of these, 319 patients fulfilled inclusion and exclusion criteria and were consecutively considered for matching. Finally, 22 patients with mono-CT-HDRBT were successfully matched to 22 patients with combined treatment of CT-HDRBT and irinotecan-TACE. One patient with combination therapy could not be matched due to a CTV of 453 ml and the unavailability of a suitable monotherapy patient with a similar lesion volume. All cohort characteristics, except the patient number with initial extrahepatic disease (combination/monotherapy: n = 8/2, p = 0.031), could be matched without significant differences. Tables 1 and 2 show baseline, disease, and treatment characteristics.
Outcomes
The median follow-up time was 10 months (25.-75. percentiles: 3.5-14.75 months). The presence of initial extrahepatic disease was no risk factor of poorer LTC (combination therapy: hazard ratio [HR] = 1.08, 95% confidence interval [CI]: 0.17-6.7, p = 0.938; mono-CT-HDRBT: HR = 1.24, 95% CI: 0.28-5.59, p = 0.779) or PFS (combination therapy: HR = 1.69, 95% CI: 0.56-5.11, p = 0.349; mono-CT-HDRBT: HR = 1.24, 95% CI: 0.28-5.59, p = 0.779) in the univariable analysis in both cohorts. In patients with mono-CT-HDRBT, the only risk factor of reduced LTC in the univariable analysis was the level of CTV (HR = 1.01, 95% CI: 1.0-1.02, p = 0.039), but no significant cut-off could be identified with ROC analysis. In the combination therapy cohort, the CTV was no predictor of poorer LTC (HR = 1.00, 95% CI: 1.0-1.01, p = 0.477) as well as any other baseline, disease, or treatment characteristic. Hence, no multivariable model could be identified. No risk factors of poorer PFS could be analyzed in both cohorts.
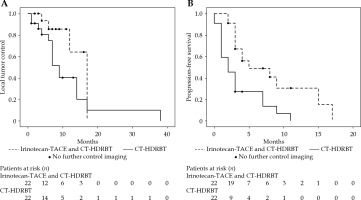
The development of local/intrahepatic progression after treatment differed significantly between both cohorts (combination therapy: n = 5/11, monotherapy: n = 15/21, p < 0.001). The median time of LTC differed considerably but not significantly, with 17 months (25.-75. percentiles: 12-17 months) after combination therapy and 9 months (25.-75. percentiles: 6-14 months) after mono-CT-HDRBT (p = 0.052). Cumulative LTC rates after 6, 12, and 18 months were 86%, 64%, and 0% for irinotecan-TACE and CT-HDRBT, and 75%, 40%, and 10% for mono-CT-HDRBT, respectively. After combination therapy with irinotecan-TACE and CT-HDRBT, the median PFS was significantly longer than after CT-HDRBT monotherapy, with 5 months (25.-75. percentiles: 3-15 months) vs. 2 months (25.-75. percentiles: 1-7 months, p = 0.002). Cumulative PFS rates at 6, 12, and 18 months were 49%, 31%, and 0% after combination therapy, respectively, and 27%, 7%, and 0% after monotherapy, respectively (Figure 4).
Safety and tolerance
Both treatments were well-tolerated. No patient died during the interventions or due to interventional complications within the follow-up period. No catheter-related major or minor complications were found in either cohort.
The blood parameter toxicity levels showed a more significant elevation of AST (p = 0.003) and ALT (p < 0.001) after combination therapy compared with monotherapy. The toxicity levels of total bilirubin increased more notably after monotherapy than after combination therapy (p = 0.034). Changes in toxicity levels of GGT, AP, INR, aPTT, and hemoglobin did not differ significantly between the groups. Tables 3 and 4 show each cohort’s baseline and post-treatment blood count and blood count toxicity results.
Table 3
Blood count results from one day before and one day after combination therapy with CT-guided high- dose-rate brachytherapy (CT-HDRBT) and transarterial chemoembolization with irinotecan or mono-CT-HDRBT treatment
Table 4
Blood count toxicity one day before and one day after combination therapy with CT-guided high-dose- rate brachytherapy (CT-HDRBT) and transarterial chemoembolization with irinotecan or mono-CT-HDRBT treatment according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0)
Discussion
In the present study, tendencies toward longer LTC and a significantly decreased local/intrahepatic progress development could be observed in the cohort, which received a combination therapy of irinotecan-TACE and CT-HDRBT compared with CT-HDRBT monotherapy. One reason might be the synergetic effects of ischemia and chemotoxicity achieved with irinotecan-TACE and irradiation effects with brachytherapy. Additionally, micrometastases surrounding the visible liver tumors, which could be deemed responsible for local tumor resurgence, may be treated more efficiently by segmental application of irinotecan-TACE than with targeted mono-CT-HDRBT. Furthermore, irinotecan has been discussed as a potential radiosensitizer, and thus may augment brachytherapy potency [19].
Additionally, a higher PFS was observed in the combination therapy cohort. This is particularly interesting because patients who received combination therapy presented with significantly more initial extrahepatic disease manifestations compared with the monotherapy cohort. An explanation could be the reduced spreading of tumor cells via the bloodstream due to an increased cytoreductive effect after both interventions compared with mono-CT-HDRBT. Furthermore, the combination therapy may enable a stronger inflammatory response, which consecutively leads to better control of extrahepatic cancer manifestations.
The cumulative 1-year LTC rates of both cohorts (irinotecan-TACE and CT-HDRBT: 64%, mono-CT-HDRBT: 40%) were higher than after monotherapy with irinotecan-TACE in a cohort of Mauri et al. (17.6%) [20]. Nevertheless, other studies noted higher cumulative 1-year LTC rates for monotherapy of CRLM with CT-HDRBT (Tselis et al. 2012/2013, 79%/73%) [21, 22], and monotherapy with radiofrequency ablation (Sparchez et al., 79%) [23]. However, the LTC results presented in this study for monotherapy with CT-HDRBT are well-comparable with the 1-year LTC rates found in studies by Nag et al. (44%) [24] and Martinez-Monge et al. (41%) after iodine-125 brachytherapy [25], while the results of our combination therapy outperformed. On the other hand, median PFS times in the literature range from 4 to 8.1 months after monotherapy of CRLM with irinotecan-TACE [20, 26, 27] and from 5 to 12.9 months after monotherapy with CT-HDRBT [9, 10, 28]. These results reflect the PFS outcome in our combination therapy cohort (5 months), while the PFS in our monotherapy cohort averaged lower (2 months).
However, as an inclusion criterion in our study was a minimum target lesion size of > 3 cm to investigate the treatment efficacy in large CRLM, this may have negatively affected the outcome of LTC and PFS compared to other studies with smaller CRLM. Further variations in baseline, disease and treatment characteristics or molecular tumor genetics (which could not be retrospectively obtained in our cohorts) complicate an accurate comparison with other studies.
None of our patients presented with catheter-related major/minor complications or interventional-associated adverse events during the follow-up period, indicating satisfactory safety profiles of both therapies. The significant increase in the toxicity levels of AST, ALT (after combination therapy), and total bilirubin (after monotherapy) has already been described by other authors after CT-HDRBT or TACE and might be related to the locoregional ischemia and inflammatory response in the acute post-interventional phase [29-34]. However, depending on the treatment modality and initial patient characteristics, other studies reported normalization of liver parameters in most patients within a more extended follow-up period, suggesting that chronic liver injury is rare with either therapy, which we were unable to control due to the short blood count follow-up period of one day before and after each procedure [31-34].
This study is limited by the retrospective cohort allocation of the group with mono-CT-HDRBT, monocentric treatment, and small sample size. Finally, it should be noted that a higher number of patients with mono-CT-HDRBT were treated with a target volume isodose of 15 Gy (n = 5) compared with the combination therapy cohort (n = 2, p = 0.083). Although the difference is not statistically significant, it may have contributed to worse LTC and PFS, given the small cohort size.