Purpose
Extra-medullary localizations of acute myeloid leukemia (AML) are called ‘granulocytic sarcoma’, ‘myeloid sarcoma’, or ‘chloroma’. Granulocytic sarcomas are mainly reported in soft tissues, bone, peritoneum, and lymph nodes [1]. We reported two patients with relapsing AML exclusively localized to the uterine cervix, which was treated successfully by combined chemotherapy and brachytherapy.
Case report No. 1
Patient No. 1 was a 25-year-old obese woman. She had no medical history. In December 2013, she was identified with bi-phenotypic acute leukemia with complex karyotype. Genotype at diagnosis was not available. Leukocyte count at onset was 4 × 103 cells/mm3, including 70% of circulating blasts. Clinical examination ruled out an extra-medullary localization. Idarubicin 12 mg/m2/day for three days, and cytarabine 2 g/m2 in eight injections led to complete remission. Thereafter, she received two cycles of idarubicin and cytarabine-based consolidation therapy, along with prophylactic intrathecal chemotherapy. In April 2014, she received pheno-identical allogeneic hematopoietic stem cell transplantation (ASCT) after myeloablative conditioning.
In January 2018, she developed metrorrhagia. Bio-psy of the uterine cervix showed numerous myeloid cells with strong CD34, without myeloperoxidase (MPO) staining, which was suggestive of granulocytic sarcoma. Staining for lymphoid markers was negative. Magnetic resonance imaging (MRI) showed a 79 mm × 64 mm × 61 mm cervical tumor extending to the upper third of vagina and the uterine body, associated with bilateral inguinal adenopathies (Figure 1A). Medullogram showed no sign of leukemic infiltration. Flow cytometry on bone marrow aspiration showed no residual disease. Next-generation sequencing of cervical biopsy revealed no mutation in ASXL1, CEBPA, DNMT3A, FLT3 (-ITD or -TKD), IDH1, IDH2, TP53, and NPM1 genes. Clinical examination showed no other extramedullary localization of AML. Donor chimerism at relapse was complete.
In April 2018, she received induction chemotherapy, composed of cytarabine 1 g/m2 twice a day for four days, amsacrine 200 mg/m2/day for three days, and dexamethasone 10 mg twice a day for three days. Therapeutic response was measured with PET-CT scan, which showed a 2 cm hypermetabolic lesion in the uterine cervix. The interpretation of this exam was limited by the lack of PET-CT scan before the initiation of chemotherapy. After the first consolidation therapy with cytarabine 1 g/m2 twice a day for three days, MRI showed a 50% regression of the cervical lesion (40 mm × 35 mm × 31 mm), with complete regression of inguinal adenopathies. A second course of cytarabine-based consolidation was administered, but the cervical lesion was still detectable after completion (size 37 mm × 36 mm × 27 mm) (Figure 1B). The patient then received pulsed-dose-rate (PDR) brachytherapy in July 2018.
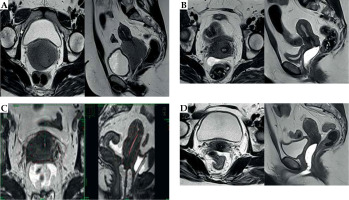
Fig. 1
MRI of the pelvis of patient No. 1. A) February 2018: Isolated relapse of acute myeloid leukemia (AML) on the uterine cervix, size 79 mm × 64 mm × 61 mm. B) July 2018: After induction and two courses of consolidation chemotherapy, persistent cervical lesion, size 37 mm × 36 mm × 27 mm. C) July 2018: Axial and sagittal view of brachytherapy plan. D) April 2019: After brachytherapy and 8 cycles of azacitidine 32 g/m2/day for 5 days, complete regression of the cervical lesion
An Utrecht interstitial applicator and six parametrial interstitial needles (3 in the right parametrium, 3 in the left) for combined intra-cavitary and interstitial brachytherapy were inserted under general anesthesia. Brachytherapy was based on a 3D computer-assisted treatment planning system (TPS) (Oncentra Nucletron, an Elekta Company). Delineation of volumes was performed on a T2-weighted MRI. Dwell time optimization was done by manually adding or removing stopping positions and adjusting dwell times. High-risk clinical target volume (HR-CTV) included gross tumor volume at the time of diagnosis and the whole cervix.
Intermediate-risk clinical target volume (IR-CTV) was delineated, and adapted from GEC-ESTRO guidelines for cervical cancers. IR-CTV corresponded to HR-CTV with a safety margin of 10 mm cranio-caudally, 10 mm laterally to the parametria, and 5 mm in antero-posterior axis. Organs at risk (OARs; bladder, sigmoid, bowel, and rectum) were systematically delineated. Physical doses were converted into a radiobiologically-weighted dose equivalent of 2 Gy/fraction (α/β = 10 Gy for tumor and 3 Gy for OARs) (EqD2 doses). 30.1 Gy in 43 pulses of 0.7 Gy was prescribed, and 38.9 Gy EqD2 to 90% of HR-CTV and 23.7 Gy EqD2 to 90% of IR-CTV were obtained. Dose constraints EqD2 to D2cm3 of the rectum, bladder, sigmoid colon, and bowel were 6.6 Gy, 28.2 Gy, 14.7 Gy, and 3.3 Gy, respectively. Irradiation was delivered through continuous hourly pulses for 43 hours. This treatment was well-tolerated, without bladder, rectal, or bowel toxicities.
Three weeks after brachytherapy, azacitidine 32 mg/m2/day for five days every month was introduced, but a new extramedullary relapse of AML developed in the left breast after two cycles. She received external beam irradiation of 30 Gy in 10 fractions, and azacitidine was administered at the same dose for a total of nine cycles, combined with one prophylactic intrathecal injection of chemotherapy and two donor lymphocyte injections. This treatment ended in April 2019. MRI performed after the second and eighth cycles of azacitidine showed persistent complete regression of the cervical lesion (Figure 1D).
Bone marrow relapse with multiple extramedullary leukemic localizations (kidney, pancreas, uterus, gluteal muscle, pericardium, adrenal glands, and bone) occurred in October 2019. Despite a combination of azacitidine and venetoclax, she died of leukemia progression in December 2019.
Case report No. 2
Patient No. 2 was a 27-year-old woman with no medical history, who had been pregnant for 10 weeks. She was diagnosed with acute myeloid leukemia revealed by cervical adenopathies in November 2014. Medullogram showed myeloid blasts with monosomy 7 and translocation (11;19) (leading to translocation between MLL and ELL). Genotype at diagnosis was not available. Biopsy of lymphadenopathy confirmed node localization of granulocytic sarcoma. Tumoral infiltration of the uterine cervix was noted during medical termination of her pregnancy. Biopsy of the lesion showed the uterine cervical localization of AML. The lesion was asymptomatic. PET-CT scan showed several granulocytic sarcomas (lymphadenopathies, paravertebral tumors, cavum, and lung), but no lesion was seen in the abdomen or pelvis at diagnosis. Cerebrospinal fluid was normal. Induction by idarubicin 12 mg/m2/day for three days and cytarabine 200 mg/m2/day for seven days led to complete remission (normal medullogram and PET-CT scan). She received two cycles of consolidation therapy with cytarabine 3 g/m2/day for three days, followed by a new PET-CT scan, which confirmed the persistence of complete remission. In April 2015, she received a pheno-identical ASCT.
Whereas blood and bone-marrow tests remained negative, a 4 cm tumor of the uterine cervix was identified in April 2018 by pelvic ultrasonography. Biopsy showed a cervical relapse of AML. Myelogram was normal. Minimal residual disease by flow cytometry on the bone marrow aspiration was not available. CT-scan showed no extra-cervical involvement. While CT-scan identified the cervical lesion, PET-CT scan showed no hypermetabolism in this site or in any other. Donor chimerism at relapse was complete.
This uterine cervix-limited relapse of AML was treated with a new induction regimen in May 2018, including cytarabine 3 g/m2 twice a day for four days, amsacrine 200 mg/m2/day for three days, and dexamethasone 10 mg twice a day for four days. MRI of the pelvis was performed one month after the induction chemotherapy and showed no lesion on the uterine cervix. Clinical examination did not reveal a cervical tumor, but the uterine cervix still seemed infiltrated on palpation. She then received first consolidation chemotherapy with cytarabine 1.5 g/m2 twice a day for three days. A new MRI of the pelvis showed no lesion on the uterine cervix or any other lesion. The uterine cervix and vagina were normal at clinical examination.
In August 2018, she received pulsed-dose-rate (PDR) brachytherapy. An Utrecht interstitial applicator and three interstitial needles in the right parametrium were inserted under general anesthesia for combined intra-cavitary and interstitial brachytherapy. As for patient No. 1, the same TPS, the same definition of target volumes, and OARs delineation were applied, and the EqD2 doses calculated. 30.1 Gy in 43 pulses of 0.7 Gy was prescribed, and 42 Gy EqD2 to 90% of HR-CTV and 20.5 Gy EqD2 to 90% of IR-CTV were obtained. Dose constraints EqD2 to the D2cm3 of the rectum, bladder, sigmoid colon, and bowel were 20.6 Gy, 26.2 Gy, 7.6 Gy, and 23.9 Gy, respectively. Tolerance was good with only mild dyspareunia, which abated with time. No bladder, rectal, or bowel toxicities were observed.
To prevent relapse, azacitidine was given from September 2018 to August 2019 (32 mg/m2/day for five days every month). In addition, she received three infusions of donor lymphocytes (October 2018, December 2018, and February 2019). From March 2019, grade 2 chronic graft versus host disease of the skin, the buccal and genital mucous membranes, and the eyes developed, along with obliterative bronchiolitis. Currently, the patient is alive without relapse despite withdrawal of all anti-leukemic treatment.
Discussion
Here, we reported the cases of two women under 30 years old with uterine cervix-limited acute myeloid leukemia relapse treated with brachytherapy along with chemotherapy.
Patient No. 1 presented with bi-phenotypic (B and M) acute leukemia, but with no cervical lesion at diagnosis. The cervical lesion was detected only at first relapse and only with myeloid phenotype. On the contrary, while a cervical lesion was found at the diagnosis of AML in patient No. 2 with several other extramedullary lesions, the relapse occurred only in the uterine cervix.
There is no established recommendation concerning the treatment of granulocytic sarcoma of the uterine cervix. Local treatment, such as surgery, may not be sufficient to offer prolonged remission, and the disease requires systemic treatment, such as chemotherapy. Systemic chemotherapy based on cytarabine and anthracycline may be sufficient to achieve complete remission [2]. In patients ineligible for intensive chemotherapy, hypomethylating agents may help in treating myeloid sarcomas, but current data are not sufficient to conclude about their real efficacy in this setting [3, 4].
Systemic treatment can be complemented by local treatment, such as external beam radiotherapy (EBRT), if the tumor is isolated [5]. Radiotherapy is appropriate to treat granulocytic sarcoma when the lesion is isolated and shows an inadequate response to chemotherapy, or when the tumor is symptomatic. The International Lymphoma Radiation Oncology Group recommends a low-dose EBRT regimen of 24 Gy in 12 fractions [6]. In this setting, EBRT may result in rapid local control. Of note, active AML in the bone marrow during radiotherapy may significantly alter local control and overall survival in patients with granulocytic sarcoma [7].
In these two reported cases, the tumor was easily accessible, and was limited in size. The patients received PDR-brachytherapy, allowing treatment to be administered rapidly (< 2 days), with no interruption in chemotherapy regimen. The total delivered dose was 30.1 Gy, with good efficacy on the cervical tumor and no significant side effects. To our knowledge, this is the first report on the use of brachytherapy in the treatment of cervix-limited granulocytic sarcoma, and it seems to be an effective rapid alternative to EBRT.
Although these findings are preliminary, these two cases show that brachytherapy may be used to treat uterine cervix-limited acute myeloid leukemia relapse. Its’ association with systemic chemotherapy can provide effective control of the cervical lesion. It seems very well-tolerated and does not delay chemotherapy.


