Introduction
Currently, colorectal cancer is always a threat to human life, and its incidence is on the rise worldwide [1, 2]. According to 2021 data, about 1.85 million people will be affected by the disease each year [3]. For patients with stages I, II, III and some stages IV who cannot be resected via colonoscopy, surgery remains the main treatment modality [4]. Compared with open surgery, the application of laparoscopic technology has improved the quality and reduced the trauma of bowel cancer surgery. By avoiding an incision in the abdominal wall, natural orifice specimen extraction (NOSE) further reduces the surgical blow to the patient and also reduces psychological stress and postoperative pain [5, 6]. In recent years, there have been reports showing no significant difference in the recent outcomes of NOSE for colorectal cancer compared with conventional laparoscopic surgery [7]. However, with the continuous development of NOSE, some concerns have been raised regarding the surgical safety of this method of specimen retrieval, especially regarding the principle of tumor-free asepsis [8]. Although some NOSE centers, including our research team, have confirmed the feasibility of this procedure through retrospective studies with small samples, and other research teams have recently affirmed the safety in terms of tumor-free asepsis, the research evidence is insufficient and lacks high-level evidence [9, 10]. The aim of this study was to analyze the differences in test results, short-term complications, and survival prognosis between the two procedures by comparing the results of bacterial culture and tumor cytology testing of peritoneal irrigation fluid, to provide objective evidence on whether NOSE of colorectal cancer is in accordance with the principle of tumor-free asepsis and to provide a theoretical basis for the development of NOSE.
Aim
To compare the bacterial culture, tumor cell detection results, short-term complications and survival prognosis between complete laparoscopic radical resection of colorectal cancer and traditional laparoscopic surgery. To add objective clinical evidence for the safety of complete laparoscopic radical resection of colorectal cancer without incision anastomosis.
Material and methods
Patients
This study prospectively and continuously collected 420 patients who underwent colorectal cancer surgery at our hospital between January 2018 and March 2022, and divided them into NOSE and N-NOSE groups according to the surgical method. Inclusion criteria: age 18–85; preoperative diagnosis of high-grade rectal cancer or distal sigmoid colon cancer; undergoing NOSE surgery or conventional laparoscopic surgery. Exclusion criteria: body mass index (BMI) > 35 kg/m2; patients with intestinal obstruction or perforation; patients with Hartmann or combined transabdominal perineal resection; patients with prophylactic fistula; patients with intermediate open abdomen; patients with distant metastases. The study received informed consent from all patients and was approved by the ethics committee (ethical approval number: LL2020397).
Preoperative preparation of the patient
All patients are started on a liquid diet 2 days before surgery and fasted with no food and water for 10 h before surgery. Appropriate fluid support is given. Oral polyethylene glycol electrolyte powders are given 1 day before surgery to help empty feces in the intestine. Metronidazole 0.5 g is orally administered 1 day before surgery, 3 times a day. Intravenous prophylactic antibiotics are given 0.5 h before surgery to prevent infection.
Surgical procedure
NOSE group surgical methods: 1) Establishment of laparoscopic surgical equipment and exploration of the abdominal cavity: after successful static suction compound general anaesthesia, a modified lithotomy position is adopted, a routine disinfection of the towel is performed, a pneumoperitoneum is established, the gas is maintained at 12 mm Hg, a poke card is placed in the five-hole method and the lens is routinely explored through the umbilicus into the abdomen. 2) Anatomical separation of the sigmoid colon: pull up the sigmoid colon cephalad to create appropriate tension in the mesentery, cut at the yellow-white junction line, enter Told’s gap through here, separate the cephalad to the root of the inferior mesenteric artery, continue freeing Told’s gap until you reach Told’s line where the sigmoid colon disappears. Remove the lymphatic lymph nodes around the root of the inferior mesenteric artery and clip the inferior mesenteric vessels using a hemostatic clip. The mesentery is alternately freed posteriorly and anteriorly in the sigmoid colon. The lateral peritoneal reflex of the colon is incised, the sigmoid colon is reversed to the right, and Told’s gap between its tether and the anterior renal fascia is freed inward and downward to the distal 5 cm of the tumor. 3) Rectal dissection: free the rectum from the pelvic side, free the rectum posteriorly to the peritoneal fold according to the principle of total rectal mesenteric excision (TME), free the anterior wall of the rectum and free it to the lower edge of the tumor 5 cm. 4) Tumor excision and specimen removal: After freeing to 5 cm below the tumor, the upper rectum, middle and lower sigmoid colon and the corresponding mesentery are removed together. After completion of dilation the distal bowel is fully flushed with iodine saline, the rectal stump is opened, the head of the anastomosis is placed in a protective sleeve and delivered into the abdominal cavity from the anus into the protective sleeve. The intestinal canal is incised at 15 cm from the upper edge of the tumor, the hooked-up head of the anastomosis is placed, the hooked up wire is clamped and adjusted in position, the intestinal canal is closed with a closure device, the hooked up wire is clamped and the head of the anastomosis is threaded through the intestinal canal. The specimen is placed in a protective sleeve and pulled slowly out of the body from the anus. 5) End-to-end anastomosis; Closure of the distal rectal stump. After adequate anal sphincter, the round anastomosis gun is placed to complete the end-to-end anastomosis. Placement of 1 pelvic drainage tube and 1 anal drainage tube. 6) The abdominal cavity is fully flushed with saline containing carboplatin and then closed. End of surgery. N-NOSE group surgical methods: In the N-NOSE group, a small incision of 5–8 cm is made in the lower abdomen for specimen removal and anastomotic tip insertion after completion of freeing, and the rest of the procedure is performed as in the NOSE group.
Methods of obtaining oncology results
All patients had their abdominal cavity flushed with saline containing carboplatin at the end of the reconstruction and 500 ml of the flushed saline was collected. 20 ml was taken from a sterile syringe and immediately sent to the bacteriological laboratory for bacterial culture. The remaining rinse was allowed to stand for 10 min and the lower rinse was centrifuged at 3,000 rpm for 7 min and the sediment smear was taken. It was fixed using 95% ethanol, given a hematoxylin-eosin stain and observed under the microscope; if suspicious tumor cells were found to be present, the result was positive for tumor cytology in the peritoneal lavage night fluid. If routine laparotomy after successful pneumoperitoneum establishment reveals tumor invasion of the plasma membrane, the flushing fluid is collected immediately after flushing with 200 ml of saline. Tumor cytology is performed in the same way and any suspicious tumor cells found are considered positive for preoperative tumor cytology.
Post-operative follow-up
To compare the long-term oncologic outcomes of the two groups, we routinely followed the operated patients up to March 2022. Patients with 2 years of follow-up were selected to plot survival curves.
Statistical analysis
A database was created in Excel, PSM was performed in R, and data analysis was performed in SPSS 22.0. Normally distributed measures were tested by the two independent samples t-test, and expressed as mean ± standard deviation. The skewed distribution measures were tested using the Mann-Whitney U-test and expressed as quartiles. Categorical variables were tested using χ2 tests, Pearson correlation coefficients were used to compare differences, and φ coefficients were used to analyze correlations and expressed as percentages. Survival rates were calculated by the Kaplan-Meier method and the log-rank test compared differences in the occurrence of adverse events between two groups of patients. Survival curves were plotted using GraphPad.
Results
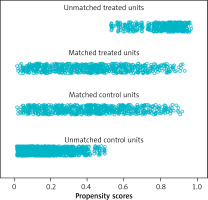
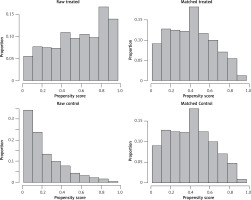
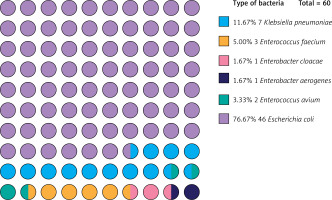
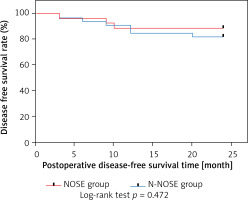
After the 420 patients included were matched 1 : 1 according to the propensity score, a total of 184 patients were entered into our study. The distribution of propensity scores between the NOSE and N-NOSE groups is shown in Figure 1. The distribution of propensity scores for the raw and matched data is shown in Figure 2. The differences in gender, BMI, T stage and N stage between the two groups of patients before PSM were statistically significant, but the differences in this baseline information after PSM were not statistically significant, as shown in Table I. There was no significant difference in the positive postoperative peritoneal washings’ tumor cytology results between the two groups after PSM (p = 0.756). The rate of positive bacterial cultures was higher in the NOSE group than in the N-NOSE group, with a statistically significant difference (p = 0.028), as shown in Table II. The bacterial species obtained by culture included Escherichia coli, Klebsiella pneumoniae, Enterococcus oryzae, Enterococcus faecalis, Enterobacter cloacae, and Enterobacter aerogenes, as shown in Figure 3. There was no significant difference in the incidence of postoperative pneumonia (p = 1.000), anastomotic fistula (p = 0.550), anastomotic bleeding (p = 1.000), intra-abdominal infection (p = 0.774), or incisional infection (p = 0.477) between the two groups, as shown in Table III. Twelve of the 13 patients who developed intra-abdominal infection also developed an anastomotic fistula, and only 1 patient developed an intra-abdominal infection without an anastomotic fistula, which occurred in the NOSE group. There was no significant correlation between the presence or absence of bacteria cultured in the patient’s peritoneal lavage fluid and the occurrence of abdominal infection (Table IV). There was no significant difference in disease-free survival between the two groups at 2 years postoperatively (p = 0.472), as shown in Figure 4.
Table I
Baseline information
Table II
Bacteriology and oncology results
Table III
Comparison of complications in the NOSE and N-NOSE groups
Table IV
Analysis of the correlation between bacteriological findings and abdominal infections
Discussion
Colorectal cancer, the third most deadly cancer worldwide, costs nearly 900,000 lives each year [11]. Surgical treatment offers the possibility of life extension for colorectal cancer patients, but also adds to their suffering: approximately 12% of patients undergoing conventional open and laparoscopic-assisted colorectal cancer surgery suffer from postoperative incisional complications [12], and many more patients suffer from significant psychological stress due to the unaesthetic incision on the abdominal wall. The application of NOSE technique cleverly avoids the problem of large abdominal wall incisions and is favored by many surgeons. Since a few small sample studies have demonstrated its feasibility in recent years [7, 13–15], NOSE surgery for colorectal cancer has become an unstoppable trend. However, as this new procedure continues to be performed, new questions are being raised: is NOSE for colorectal cancer truly consistent with the principle of tumor-free asepsis and does the NOSE technique have an impact on the long-term prognosis of patients? [8, 14] The current research evidence on this aspect is not yet sufficient. In this study, we investigated complete laparoscopic radical resection of colorectal cancer without incision anastomosis using available case resources.
In this study, PSM was used to match the data of the two groups of patients [16]. Theoretically, tumor location in the superior rectum or sigmoid colon does not have an impact on bacteriological and oncological outcomes or prognosis, so tumor location was not used to calculate the propensity score in this study. After propensity score matching, a total of 92 cases in the NOSE group and 92 cases in the N-NOSE group were successfully paired and included in the study, and baseline information in both groups improved significantly compared with pre-matching.
This study analyzed the complications observed in both groups. The incidence of pulmonary infection, anastomotic bleeding, anastomotic fistula, intra-abdominal infection and surgical incision infection was not increased in the NOSE group relative to the N-NOSE group. NOSE had a positive impact on the prevention of pulmonary infections due to less postoperative pain, earlier bedtime, greater activity, improved respiratory amplitude, and more fluid sputum evacuation in this group [5, 6, 17]. We believe that NOSE may reduce the incidence of postoperative pulmonary infections in patients, which warrants further study in a larger sample size.
Surgical site infections (SSI) are more common in patients undergoing colorectal surgery and are broadly classified as incisional and organ/space infections according to the updated definition of SSI by the Centers for Disease Control in 2003 [18]. Incisional SSI is rare after NOSE because only 5 trocar puncture incisions about 1 cm in length are left on the abdominal wall. In this study, 92 patients in the NOSE group did not develop incisional SSI. We believe that the non-significant difference in the incidence of incisional SSI between the NOSE group and the N-NOSE group is due to the small sample size. SSI of the organ/space, or intra-abdominal infection as described in this article, is the more serious complication, and its most common cause is postoperative anastomotic fistula [19]. However, colorectal NOSE surgery opens the intestinal canal directly into the abdominal cavity during GI reconstruction, and the use of a lumpectomy sleeve to drag the specimen out via the anus does not guarantee that the sleeve is completely closed in the abdominal cavity, which may also cause contaminants to enter the abdominal cavity under the compression of the anal sphincter. Can this also lead to intra-abdominal infections like an anastomotic fistula? To address this issue, this study was conducted carefully in conjunction with the bacteriological findings of the peritoneal lavage fluid. The results showed that 46 (76.7%) of the 60 positive cultures for bacteria were Escherichia coli, the rest were common bacteria in the intestine (23.3%), and no bacteria from outside the intestine were present. The bacterial positivity rate was higher in the NOSE group (42.2%) than in the N-NOSE group (25.0%), which is similar to the existing reported rate of abdominal contamination in NOSE procedures [9]. Reassuringly, even though the rate of bacterial contamination of the peritoneal cavity was significantly higher in the NOSE group, it did not increase the risk of postoperative intra-abdominal infection. Based on the occurrence of postoperative intraperitoneal infection in those with a contaminated peritoneal cavity, this study successively performed correlation analysis for all matched patients, the NOSE group and N-NOSE group, respectively, and revealed no significant correlation between the rate of bacterial contamination of the peritoneal cavity and the rate of intraperitoneal infection. This suggests that even though NOSE increases the rate of peritoneal cavity contamination, it does not increase the risk of intra-abdominal infection. Twelve of the 13 patients who developed intra-abdominal infection in this study also had anastomotic fistulas, which likely caused the intra-abdominal infection directly from the anastomotic fistula. Bacterial colonization of the abdominal cavity causing intra-abdominal infection is certain to occur under certain conditions. All our surgical patients received strict bowel preparation before surgery, and intravenous prophylactic antibiotics were administered 0.5 h before surgery and 3 days after surgery. These measures have played a positive preventive role in reducing the rate of abdominal infection.
In this study, adequate distal margins were secured in all patients, and postoperative pathological testing confirmed negative peri-annular margins. Both the NOSE and N-NOSE groups had patients with positive oncology test results, but there was no significant difference in the results between the two groups. This suggests that NOSE as a modality did not increase the risk of patients having tumor cells shed into the abdominal cavity. Our surgical team has accumulated rich experience in colorectal NOSE surgery and has also performed NOSE surgical treatment on some patients with stage T4. We found that for the NOSE procedure, the detection of tumor cells in the irrigation fluid was not entirely due to the shedding of tumor cells as a result of the surgical operation; it was most likely due to local infiltration of the tumor or lymph node metastasis so that tumor cells were already shed into the abdominal cavity preoperatively. This is based on our innovative approach of performing intraoperative laparotomy in patients with tumor invasion to the plasma membrane immediately after the establishment of the pneumoperitoneum and collecting the laparotomy fluid for examination, and also detecting tumor cells. Thus, it appears that the possibility of tumor implantation and metastasis is equally present whether the specimen is taken through an adjuvant incision in the abdominal wall or through the natural cavity. In the few available studies of surgical tumor-free asepsis in NOSE, no preoperative peritoneal irrigation fluid exfoliation cytology has been reported.
Because of the short follow-up period, we studied local recurrence and death as positive events. The study showed that the 2-year postoperative disease-free survival rate was 88.89% and 81.82% in the NOSE and N-NOSE groups, respectively, and there was no significant difference in the 2-year postoperative disease-free survival rate between the two groups. It can be assumed that even though the tumor cell test result is positive, it does not affect the long-term outcome of patients in the NOSE group, and the 2-year disease-free survival rate after surgery is at least comparable to that of conventional surgery. This is in line with the findings of existing studies [9, 13, 20]. In 2019, Liu et al. also demonstrated through a meta-analysis that NOSE for colorectal cancer can achieve satisfactory oncological outcomes [21]. For T4 patients in whom there is only invasion of the plasma membrane, NOSE still has some advantages: the pain of enduring a large abdominal wall incision is avoided with the same outcome.
Of course, this study has some limitations: it was not a randomized controlled study; the sample size was not large enough; the effect of unknown bias could not be controlled for; the follow-up period was short; and only NOSE surgery for high-grade rectal and distal sigmoid colon cancer was studied.