Introduction
Esophageal cancer (EC) is an important public health problem [1] which was identified as the sixth leading cause of cancer death worldwide [2]. Due to the quick spread and high mortality rate, EC cancer is also considered as one of the most serious tumor diseases in the world [3]. Furthermore, EC is the eighth most common malignant cancer (3.2% of all cancer cases). The highest rates of this disease have been recorded in East Asia, East and South Africa, while the lowest rates have been observed in West Africa [4]. Studies have shown that EC has been diagnosed more in men than in women in the last 40 years, and the incidence and strong predominance of this disease among men have slightly increased [5]. Histologically, there are two main types: adenocarcinoma of the esophagus (ACE), which is prevalent in western populations, and squamous cell carcinoma (SCC), which is most common in Asian populations [6, 7]. In line with the risk factors for EC, according to studies, there are risk factors for esophageal squamous cell carcinoma (ESCC), which are alcohol and tobacco consumption, hot drinks, poor oral hygiene, genetic mutation in enzymes that metabolize alcohol, burning injuries, exposure to chest radiation, nutritional deficiencies, low socioeconomic status, as well as two disorders called tylosis and achalasia [8, 9]. Also, there is direct contact between carcinogens in tobacco and the esophageal mucosa, which further contributes to the risk of esophageal squamous cell carcinoma. There are various therapeutic options for EC, including chemotherapy (CT) [10–13], radiotherapy (RT) [10], surgery [10] and, for better diagnosis or more advanced types, endoscopic procedures [11–14]. Notably, these mentioned therapeutic methods are associated with some serious disadvantages and many side effects for the patients. For instance, chemotherapy impacts regular cells and the immune system, potentially causing cancer recurrence. Hence, it is crucial to explore new therapeutic drugs without such complications [10].
Immunotherapy stands out as an innovative option, with numerous reported advancements. The mode of action of monoclonal antibodies (mAbs) is promoting the immune system, targeting specific markers of EC tumor cells. Furthermore, these mAbs such as pembrolizumab, ramucirumab, trastuzumab nivolumab, etc., in different treatment lines could be applied.
The first line of treatment is the CT method, which can delay tumor growth and reduce the burden of tumor mass and improve the 5-year survival of cancer patients [15–17]. In line with the treatment of EC, it has been shown in research that in addition to the surgical approach to this tumor, whenever CT, RT and immunotherapy are used, they can have advantages for the treatment of this cancer [18]. Studies have shown that patients with EC who undergo CT have reduced skeletal muscle mass or sarcopenia as complications of infection [19–22]. Another side effect of CT and chemoradiotherapy (CRT) is loss of appetite, deterioration of nutritional status, diarrhea and neutropenia [23–26]. In addition, treatment with CT and methods based on it can damage DNA and activate the complex cell signaling network, which itself causes the cell cycle to stop and initiates apoptosis [15]. As mentioned above, in order to enhance the quality of life and increase the survival of patients with EC, immunotherapy is a suitable candidate for patients with EC [27, 28]. However, the cancer cells of this lethal tumor after CT and the initial response to that treatment often develop multidrug resistance, which leads to recurrence of the tumor [16, 17].
According to researchers, the main goal of immunotherapy to treat cancer is to overcome inhibition of the immune system by the tumor. Unlike CT, immunotherapy and targeted therapies are designed to attach specific molecules that are maximally expressed by the tumor such as HER-2, CEA, CA19-9 and CA15-3 [29]. Several studies show that immunotherapy has made a large change in the treatment of EC, and its outcomes include better quality of life, tolerable toxicity, high effectiveness, and improved survival rate [11, 13]. Furthermore, immunotherapy is effective in the treatment of other solid tumors and has led to the recovery of many cancer patients [30]. Programmed cell death (PD) and its ligand are expressed highly on the surface of tumor cells and they can suppress T cell immune responses. Some studies on programmed cell death-1 (PD-1)/programmed death-ligand 1 (PD-L1) ligands indicated the presence of PD-1 as a protein on the surface of T lymphocyte cells. This receptor provides an inhibitory signal which results in the highest expression of PDL-1 ligand on tumor cells. The outcomes of this interaction lead to inhibition of the activation and performance of T lymphocytes [31]. Therefore, immunotherapy of EC with the effect on PD-1/PD-L1 has illustrated a remarkable correlation with cancer care [32]. In this study, a comprehensive review of the latest findings related to immunotherapy in EC was performed.
Pembrolizumab mAb in esophageal cancer
There are complementarity-determining regions (CDRs) derived from mouse anti-human PD-1 monoclonal antibodies, and framework and constant regions derived from human IgG4, in which the amino acid proline is substituted for amino acid residue 228, a glycoprotein with the molecular weight of 149,000 Da composed of four heavy chains (447 amino acid residues each) and two light chains (218 amino acid residues each) [33].
Pembrolizumab targets PD protein 1 and is a novel human mAb. This mAb can target PD-1 receptors with high affinity in both antigen-presenting cells (APCs) and tumors; thus, PD-L1 and PD-L2 are inhibited in the PD-1 pathway. In a tumor microenvironment, pembrolizumab can reactivate antitumor T lymphocytes and stimulate them to become more effective. Pembrolizumab has been approved by the US Food and Drug Administration (FDA) for patients with metastatic ESCC. Despite their infrequency, pembrolizumab can cause some adverse reactions. However, pembrolizumab does not have as many adverse effects as conventional cytotoxic drugs. The first-line use of pembrolizumab in combination with standard CT and the locally advanced use of pembrolizumab combined with definitive chemoradiation are currently being evaluated. An EC trial using pembrolizumab monotherapy showed superior survival to standard CT [34]. There is also a positive overall score of 10 for pembrolizumab monotherapy in patients with advanced ESCC. Treatment of advanced EC patients with pembrolizumab plus CT has a higher efficacy (45%) as compared to treatment with CT alone (30%) [33, 35] (Fig. 1).
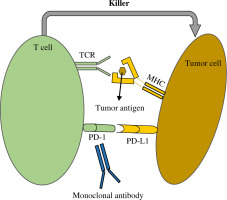
Fig. 1
Mode of action of monoclonal antibody. Anti-PD-1/PD-L1 antibody suppresses interaction between PD-1 and PD-L1. Generally, tumoricidal function of T cells can be hindered by PD-1 and PD-L1. However, monoclonal antibodies overcome this inhibitory effect. Consequently, T cells kill the tumor cells by receiving tumor antigens from MHC via TCR. PD-1 – programmed cell death-1, PD-L1 – programmed cell death ligand-1, TCR – T cell receptor, MHC – major histocompatibility complex
Recent studies have shown that checkpoint the inhibitors nivolumab and pembrolizumab are effective in advanced EC patients. There are still some concerns following the current results of large clinical trials. This is even though they have shown high efficacy, tolerable toxicity, and promising survival rates [35]. Despite the obtained results, it is still not clear which combination therapy can have the greatest therapeutic effect for advanced EC patients.
A trial with treated patients with EC found that pembrolizumab monotherapy might prove efficacious. In addition, the present review study revealed that pembrolizumab provides better efficiency in patients with ESCC than in patients with esophageal adenocarcinoma (EAC), suggesting that therapeutic approaches for ESCC and EAC need to be revised. As well as the combined positive score (CPS) (lymphocytes, macrophages, and number of PD-L1-positive cancerous cells divided by the total number of tumor cells) this trial evaluated the expression of PD-L1 and it was found to be indicative of pembrolizumab efficacy [36, 37].
Therapeutic effect of atezolizumab mAb in esophageal cancer
Atezolizumab is a synthetic humanized IgG1 mAb that targets the PDL-1 molecule. This drug became the first FDA-approved mAb to treat urothelial cancer in 2019. The structure of the antibody demonstrates that atezolizumab can bind to PD-L1 with a discrete heavy and light chain orientation and it suppresses the interplay of PD-1 to PDL-1 via interfering with PD-1 attachment to PDL-1. Additionally, PD-L1-expressing cancer cells can interact with T cells expressing PD-1 receptors, impairing the immune response against tumors. PD-L1 and PD-1 blockade triggers a T cell immune response in response to cancer. PD-L1 syndrome can be treated with several medicines, including durvalumab and atezolizumab [38]. The presence of PD-L1 expression appears to be associated with the ability to predict the response to treatment, but its ability to predict an anti-tumor response remains to be explored [39].
According to a multicenter phase II proof-of-concept study, atezolizumab (Tecentriq) was demonstrated to be an effective and safe treatment for patients with locally advanced ESCC following chemotherapy. When atezolizumab was combined with CT, the complete response (CR) rate was obtained. Achieving a CR refers to the absence of all detectable cancer after treatment. It is important to note that a complete response does not guarantee a cure, but it represents the best possible outcome.
An increase in expression of PD-L1 and activation of epidermal growth factor receptor (EGFR), extracellular signal-regulated kinases (ERK), and PD-L1 was observed in ESCC cell line cultures treated with CT. Some studies used an EGFR inhibitor (erlotinib) as well as a MAPK/MEK inhibitor (AZD6244) after CT to prevent upregulation of PD-L1 as a result of CT. Consequently, CT combined with anti-PD-L1 immunotherapy is likely to be effective in treating ESCC [40–42]. It was also reported in a phase III study that atezolizumab monotherapy was as effective as regorafenib, a tyrosine kinase receptor blocker, for chemotherapy-refractory metastatic colorectal cancer (mCRC) as compared to atezolizumab and cobimetinib (MEK1/2 inhibitor) [43]. Also, in patients who received atezolizumab, 55.1% were given sorafenib. Atezolizumab has been shown to be a significant improvement over sorafenib; hence it may replace it as a first-line treatment in the near future. There are a number of other clinical trials currently underway involving anti-PD-1/PD-L1 antibodies combined with tyrosine kinase inhibitors (TKIs) or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) antibodies [44, 45]. In this regard, atezolizumab was well tolerated and had an acceptable safety profile in heavily pretreated patients with advanced EC [46].
Interestingly, CROSS-based neoadjuvant chemoradiotherapy (nCRT) in combination with atezolizumab has never before been used to treat resectable esophageal adenocarcinoma (rEAC). Atezolizumab was found to be feasible for the study population, with 85% of the participants receiving the drug for its entire duration [47]. During the trial, atezolizumab was combined with nab-paclitaxel and carboplatin for neoadjuvant treatment of rEAC cancer. As a result, it is possible that paclitaxel or paclitaxel and atezolizumab caused the dermatologic reactions [48]. Therefore, the addition of atezolizumab to conventional nCRT for rEAC did not adversely affect the surgical outcomes [47].
Therapeutic effect of avelumab in esophageal cancer
Avelumab (Bavencio) is another anti-PD-L1 mAbs which is a fully human antibody (IgG1) and it has demonstrated promising results against tumors and potential safety [49].
The FDA has approved three monoclonal antibodies targeting PD-L1: durvalumab, atezolizumab, and avelumab. According to findings presented at the 2019 ASCO Conference, neoadjuvant CT and RT with avelumab are effective and safe treatments for resectable EC and gastroesophageal junction tumors. During the stage I/II randomized trial, there was a relatively small sample size. There is an urgent need to study neoadjuvant CRT combined with avelumab in the treatment of patients with EC and gastroesophageal junction tumors [50]. Avelumab was approved for use in Asia and subsequently it was approved in the United States too, since this trial demonstrated its effectiveness [51, 52].
Treatment of patients with gastric cancer/gastro-oesophageal junction cancer (GC/GEJC) can be achieved with avelumab. Interestingly, avelumab has approval for metastatic urothelial carcinomas among other solid tumors [49, 53]. In patients with advanced GC/GEJC who received avelumab as part of the phase I JAVELIN trial it was found that the drug was effective as either a second-line maintenance treatment or first-line maintenance treatment [54]. Actually, it was the primary objective of that study to show that avelumab provides higher overall survival as compared to CT. To indicate the priority of avelumab over CT, the drug was compared with CT. In addition to the primary objective, secondary objectives included the evaluation of progression-free survival (PFS) and objective response rate (ORR) based on assessments by the independent review committee (IRC) [52].
Additionally, when compared with CT, avelumab has a superior safety profile. The study conducted by Bang et al. [52] also indicated that avelumab was associated with fewer treatment-related adverse events (TRAEs) than CT. As a result of these findings, avelumab is well tolerated when combined with CT in patients with GC/GEJC, suggesting that it may be used in late stages of the disease for both combination therapy and maintenance treatment. The optimal strategy for incorporating checkpoint inhibitors earlier in the treatment process for patients with advanced GC/GEJC remains unclear; replacement anti-PD-1/PD-L1 therapeutic approaches are required [52]. Eventually, there was a durable response to avelumab treatment in patients who were heavily pretreated with this drug regardless of whether the tumors expressed PD-L1 [55].
Nivolumab antibody: Neoadjuvant for recurrent esophageal cancer
The fully human mAb nivolumab is a drug that inactivates PD-1 and triggers antitumor activities. Researchers found that nivolumab monotherapy significantly improved survival when compared with taxane as a second-line treatment for advanced ESCC patients. Moreover, nivolumab monotherapy after neoadjuvant CT followed by surgery improved disease-free survival significantly compared to placebo in patients with resectable EC and residual pathology.
Nivolumab is the recommended treatment for patients with advanced ESCC following neoadjuvant CT and surgery. First-line treatment options include CT, RT, and neoadjuvant CT containing nivolumab, as well as cytotoxic agents and nivolumab combined with other drugs. There should be improvements in the clinical protocols for EC clinical trials in order to improve the clinical outcomes. In addition to targeting protein 1 of the PD pathway, nivolumab is also an IgG4 mAb to target protein 2 of the PD pathway. Based on the results of the ATTRACTION3 phase III trial, the FDA approved nivolumab monotherapy as a treatment option for patients who have progressed following fluoropyrimidine and platinum-based systemic CT for advanced, recurrent, or metastatic ESCC. The efficacy of adjuvant nivolumab monotherapy in the CheckMate 577 trial was greater than that of placebo in patients who did not achieve a pathologic CR after neoadjuvant CT [56].
The standard course of treatment for ESCC is neoadjuvant CT followed by surgery. Nivolumab was successfully used to treat metastatic ESCC. The effectiveness and safety of nivolumab as a neoadjuvant therapy for ESCC have not yet been evaluated [57].
Despite widespread use of neoadjuvant CT and RT, the recurrence rate is high in both GEJC and EC. In a global, randomized, double-blind phase III trial, nivolumab was used as adjuvant therapy for patients with GEJC and patients at high risk of recurrence following nCRT and gastroesophageal junction resection [58]. Results of this clinical trial study showed that nivolumab adjuvant therapy remarkably prolonged disease-free survival. In an early phase II trial, CheckMate 577, the aim was to determine whether nivolumab adjuvant therapy could be effective in improving disease-free survival among patients with resectable, locally advanced esophageal and GEJC [59].
Despite poor prognoses, nivolumab resulted in an improvement in disease-free survival, reducing recurrence or mortality risk by 31%, and extending median disease-free survival by twice as long as the control group. The hazard ratio for nivolumab over placebo was considerably higher for most prespecified subgroups, including histology (SCC and ACE) and lymphoma status [60]. Notably, in the treatment of patients with advanced gastroesophageal cancer who have previously been treated as well as those who have low levels of PD-L1 expression in tumor cells, nivolumab does not produce any differences in clinical benefit [61, 62]. Combining PD-L1, PD-L2 and PD-L1 expression enhances the effectiveness of checkpoint inhibitors more efficiently than the expression of PD-L1 alone [63].
The CheckMate 577 trial revealed that adjuvant nivolumab was equally effective regardless of the level of PD-L1 expression within tumor cells. The hazard ratio for disease recurrence or death among patients with EC was greater than that for patients with GEJC. After treatment with nivolumab, people with EC and GEJC had similar disease-free survival rates. However, those who received a placebo had a longer median disease-free survival than those with EC, and individuals with GEJC had an even longer median disease-free survival. As a result of nivolumab-adjuvant therapy, recurrences and deaths were 26% lower, as were metastasis-free survival rates, which were 10.7 months longer than those without nivolumab treatment [60]. Moreover, there are positive findings in the CheckMate 577 trial, in which nivolumab has shown promising results in adjuvant treatment of esophageal and GEJCs following melanoma [64].
Nivolumab adjuvant therapy has demonstrated similar safety profiles in other studies in patients with gastroesophageal cancer and other solid tumors [61, 62, 64-66]. The trial regimen was discontinued because of serious adverse events or adverse outcomes associated with nivolumab. Patients with resected EC or GEJC after neoadjuvant CT were significantly more likely to survive disease-free after nivolumab adjuvant therapy. Nivolumab exhibits a similar safety profile to other types of solid tumors [60]. As matter of fact, although nivolumab is effective in the patients, its serious adverse complications are also reported. Therefore, the use of nivolumab was not continued.
Camrelizumab monoclonal antibody in esophageal cancer
As a selection of mAb against IgG4-kappa PD-1 with humanized chains, camrelizumab (SHR-1210) could be applied to treat PD-1 mutations. The treatment of patients with relapsed or refractory classic Hodgkin lymphoma was conditionally approved through an approved clinical trial conducted by Jiangsu Hengrui Medicine Co., Limited in 2019 [67–69].
Immunotherapy, particularly anti-PD-1 antibodies, has shown improved overall survival in patients with advanced solid tumors after treatment with immune-related adverse events (irAEs). It has been reported that the most common serious adverse event associated with camrelizumab is reactive cutaneous capillary endothelial proliferation (RCCEP). Despite its widespread occurrence in the skin, oral RCCEP has rarely been reported. It will also become common for oral RCCEP to be used in solid tumors in addition to camrelizumab. Surgery and ligation treatment of RCCEP are both associated with a good prognosis [70].
Three additional indications were approved of camrelizumab in China in 2020: (a) patients with malignant hepatocellular carcinoma who had previously received sorafenib and/or oxaliplatin as systemic CT; (b) in combination with pemetrexed and carboplatin to treat locally advanced or metastatic nonsquamous non-small cell lung cancer (NSCLC) without EGFR or ALK mutations; or (c) as a second-line treatment for locally advanced or metastatic ESCC.
As a first-line therapy, definitive RT plus camrelizumab has been evaluated for patients with locally advanced ESCC who were not eligible for or refused concurrent chemotherapy (CCRT). Efficacy of RT as a first-line treatment for metastatic ESCC has been assessed in combination with camrelizumab mAb against PD-1. Locally advanced ESCC was successfully treated with camrelizumab and RT. Further research seems to be necessary in order to identify clinically useful biomarkers. There was some evidence that RT plus camrelizumab was safe and effective as a first-line treatment for patients with locally advanced ESCC. This study demonstrated that a novel first-line treatment combination combining RT and camrelizumab showed promising antitumor activity and manageable toxicity in patients with locally advanced ESCC [67–69]. Patients with advanced or metastatic ESCC face a challenging diagnosis with limited therapeutic options. After undergoing CT, camrelizumab was compared to the CT employed by investigators in patients previously treated with this antibody to determine its efficacy and safety. The researchers found a remarkable enhancement in survival for patients with ESCC treated with camrelizumab compared with CT. It was also possible to manage the product’s safety profile. The treatment may be considered as a standard option for the treatment of patients with esophageal squamous cell carcinoma as a second line of treatment [71].
As a treatment adoption for advanced or metastatic malignant cancers, camrelizumab as a novel inhibitor of PD-L1 is being investigated, including ESCC, GEJC, hepatocellular carcinoma, nasopharyngeal cancer and NSCLC. The results of clinical trials revealed the remarkable increase in overall survival by a spectrum of irAEs due to its hyper-activation of the immune system. It is often associated with digestive disorders, liver disorders, endocrine disorders and skin disorders. Actually, it depends on the etiology of the primary tumor as to what skin lesions occur. On the other hand, pembrolizumab and nivolumab also have irAEs associated with them, but camrelizumab’s most common irAE is RCCEP, which usually occurs on skin that is exposed to it. It occurs most commonly on the head, face or trunk of the body and is one of the most common adverse reactions to camrelizumab. The side effects of camrelizumab are likely to include RCCEP in about 80% of patients. RCCEP can be easily confused with other disorders, such as angioma, epulis, or even tumor; however, there are a few cases reported with RCCEP in the eyes, nose, and oral cavity [70, 72].
Tislelizumab antibody
In an effort to develop a new immunotherapy and anti-neoplastic therapy, BeiGene is developing tislelizumab, a monoclonal IgG4 antibody that targets the PD-1 receptor on human cells [73]. There is an approval for the PD-1 mAb tislelizumab that has high affinity and specificity, for the treatment of advanced NSCLC, classical Hodgkin lymphoma, and metastatic PD-L1-high urothelial carcinomas [74, 75].
In patients with advanced unresectable or metastatic ESCC, there is already significant evidence that immune therapy utilizing tislelizumab can improve survival over CT [76]. As well as its preliminary anti-tumor properties, tislelizumab has been demonstrated to be safe among cancer patients. Furthermore, tislelizumab has been applied in ESCC patients and the current evidence indicates that immune checkpoint inhibitors (ICIs) can be used as neoadjuvant therapies. We hypothesized that tislelizumab combined with CT could provide a valuable treatment option for surgically resectable ESCCs [75, 77]. To assess the efficacy and safety of tislelizumab in combination with CT for patients with resectable ESCC, some studies examined tislelizumab plus CT as neoadjuvant therapy. Treatment-naive patients were given carboplatin, nab-paclitaxel, and tislelizumab as neoadjuvant therapies. In the mentioned study, the primary outcome was the major pathological response (MPR) of the patients undergoing surgery. It has been shown that tislelizumab plus CT is an effective neoadjuvant treatment for resectable ESCC with high rates of resection of MPR, pathological complete response (pCR), and R0 lesions with acceptable tolerability [78].
Another study, the phase III trial RATIONALE-306 (NCT03430843), compared tislelizumab monotherapy with CT (taxane or irinotecan) for the treatment of metastatic or recurrent ESCC. The phase III trial RATIONALE-302 is currently being conducted in patients with metastatic or recurrent ESCC who have not previously received CT (5-FU + platinum or paclitaxel + platinum). It is currently being evaluated whether CT is effective in conjunction with nivolumab and pembrolizumab as a first-line treatment (CheckMate 648, KEYNOTE-590) as well as whether CT combined with tislelizumab is effective as a second-line or first-line treatment (NCT03783442, NCCT03430843) [79]. This phase II study (NCT03469557) evaluated the tolerability, safety and antitumor activity of tislelizumab against PD-1 in combination with CT in patients with locally advanced or metastatic ESCC or GEJC adenocarcinoma. With manageable side effects, tislelizumab plus CT has been reported to be effective in treating metastatic ESCC or gastric/gastroesophageal junction (G/GEJ) adenocarcinomas (ACCs) [80–82].
Durvalumab in esophageal cancer
Another anti-PD-L1 mAb is durvalumab, applied in patients with NSCLC. In the PACIFIC clinical trial, the researchers compared the effects of durvalumab on patients suffering from locally advanced NSCLC. In all patients, the primary endpoints were the probability of survival and overall survival. When compared to placebo, durvalumab significantly improved the life expectancy of patients with locally advanced NSCLC [79, 83].
Additionally, immunotherapy with durvalumab has been shown to be promising in early clinical trials in patients with GEJC [84]. The phase III study will utilize neoadjuvant-adjuvant durvalumab plus FLOT (5-fluorouracil-leucovorin-oxaliplatin-docetaxel) CT followed by adjuvant durvalumab monotherapy for patients with resectable GEJC [84]. Durvalumab proved to be an effective consolidation therapy for patients undergoing platinum-based chemoradiation therapy for stage III NSCLC in the phase III PACIFIC study (NCT02125461) [85]. According to updated results from the phase III CASPIAN study (NCT03043872), durvalumab combined with etoposide and cisplatin/carboplatin demonstrated a sustained overall survival (OS) benefit in patients with extensive-stage small-cell lung cancer (ES-SCLC) [86, 87]. The combination of a single priming dose of tremelimumab, a fully human mAb that targets CTLA-4, plus durvalumab in STRIDE displayed superior efficacy vs. sorafenib, and durvalumab monotherapy was noninferior to sorafenib. Both treatments had a favorable benefit-risk profile vs. sorafenib in patients with unresectable hepatocellular carcinoma. Based on improved immunotherapeutic responses in the presence of CT, durvalumab combined with FLOT may be considered for patients with resectable GC/GEJC [88–90]. In the randomized, open-label, phase III CheckMate 649 study, nivolumab in combination with CT demonstrated significant improvements in OS and an acceptable safety profile vs. CT alone as first-line treatment for advanced GC/GEJC/EAC [91].
Interestingly, durvalumab not only has the capability of enhancing the function of effector T-cells, but it has also been shown to be effective at eliminating tumor cells by blocking specifically the interaction between PD-L1 and PD-1 [85, 92].
In addition to NSCLC, durvalumab is effective in treating a variety of other tumor types. Moreover, its safety profile is manageable [93].
GC/GEJC that has been resectable and metastatic is being treated with durvalumab, which is being developed based on preliminary data from two early phase clinical trials [94, 95]. Based on these early-phase studies and observations in other tumor types, durvalumab treated patients with GC/GEJC in conjunction with FLOT had better clinical outcomes than patients treated with FLOT alone. Although technological advances have been made in the treatment of GC/GEJC, the 5-year OS rate for patients with resectable tumors remains suboptimal, and it will be necessary to develop new treatments. The use of durvalumab for the treatment of patients with metastatic or recurrent GC/GEJC has shown promising anti-tumor effects. With the combination of FLOT cytotoxic CT and durvalumab, patients with resectable GC/GEJC may experience an improved outcome. A phase III study will assess whether combining perioperative durvalumab with FLOT CT, followed by durvalumab alone, is effective for resectable GC/GEJC patients. The results of this clinical trial study indicate that ICIs can be used as part of a combination CT regimen in neoadjuvant-adjuvant cancer treatment [84].
Ipilimumab antibody; CTLA-4 inhibitor
Immune checkpoint inhibitors are increasingly used to treat cancer due to their ability to reactivate immune cells. Ipilimumab (anti-CTLA-4 antibody), nivolumab (anti-PD-1 antibody), pembrolizumab (anti-PD-1 antibody), atezolizumab (anti-PD-L1 antibody), and durvalumab (anti-PD-L1 antibody) are a number of ICIs that are approved for cancer treatment [96, 97]. Ipilimumab (Yervoy, Bristol-Myers Squibb) binds to the human and canine CTLA-4 and suppresses its interaction with CTLA-4 ligand. For the initial production of the mAb, transgenic mice (HC2/KCo7 strain) expressing the CTLA-4 extracellular domain were used. Research conducted in phase I used antibodies derived from hybridomas 10D1, whereas research conducted in phase II employed Chinese hamster ovary (CHO) cells transfected with vectors containing heavy and light chains [98]. T cells are capable of upregulating CTLA-4 receptors, which are also targeted by anti-bodies targeting ICIs. In addition to suppressing T cells, CTLA-4 interacts with APCs in order to accomplish this function. Consequently, CTLA-4 is blocked using, for example, ipilimumab, thus promoting T cell activation [39].
Cancer immunotherapy has been tested with mAbs that target CTLA-4. T-cell-mediated responses are inhibited by CTLA-4. First ipilimumab was found to improve survival in patients with unresectable or metastatic melanoma. Currently, only a combination of nivolumab and ipilimumab has been approved for the treatment of melanoma and other advanced or metastatic solid tumors. The mAb combination may provide better clinical efficacy, but there is an increased risk of irAEs, which can result in patients discontinuing treatment even when they respond to it. Researchers have developed anti-CTLA-4 antibodies that are proteolytically activated in tumor microenvironments, as well as bispecific molecules that target both CTLA-4 and PD-1, which are both expressed by tumor-infiltrating T cells. Additionally, these molecules are less toxic to normal tissues than tumor cells in addition to stimulating immune responses against tumors [99, 100].
Since PD-1 and CTLA-4 are blocked by ipilimumab and nivolumab simultaneously, this combination provides improved clinical efficacy. However, IrAEs are more frequent and severe, as the multispecific antibodies (msAbs) inhibit CTLA-4-expressing Treg cells in normal tissues, resulting in a breakdown in immunological tolerance. Generally, severe irAEs may require treatment modifications, such as reducing doses, ceasing treatment, or modifying immunosuppressive therapy [101].
The use of ICIs in cancer treatment has transformed how tumors are treated because they reactivate the immune system in order to eradicate tumor cells. Since anti-CTLA-4 antibodies perform in different directions, when combined with anti-PD-1 (or anti-PD-L1) antibodies, they can have synergistic effects against a wide range of cancer types. It is possible, however, that irAEs may be more frequent in circumstances where there is a strong immune response. In a patient with advanced ESCC receiving nivolumab in combination with ipilimumab, immune-mediated liver damage occurred [102, 103].
Combination therapy or singular immunotherapy?
Several immuno-oncology combination treatments have been tested in first-line trials for treating advanced EC, but the optimal immuno-oncology combination treatment has not been identified [104].
Pembrolizumab
In studies conducted by researchers, it has been determined that pembrolizumab combined with 5-fluorouracil and cisplatin (PPF) is a superior first-line treatment for EC compared to 5-fluorouracil and cisplatin (PF). In spite of its high cost, however, there is still debate about its value when compared with other forms of competition.
PPF may not be as cost-effective as PF for EC therapy based on the economic perspectives of the USA and China. However, patients with EC and PD-L1 CPS ≥ 10 may gain the most life-years from initial PPF treatment [105].
In Japan, advanced EC treatments are unmet needs. The results of a subgroup analysis in KEYNOTE-181, a randomized, open-label trial involving Japanese patients, were presented. In patients with advanced or metastatic EC who have failed standard first-line therapies, pembrolizumab is used in combination with CT as a second-line treatment. Japanese patients with advanced EC were found to benefit from pembrolizumab as a second-line treatment over CT when it was used as a second-line treatment [106].
KEYNOTE-181 evaluated pembrolizumab as a second-line therapy against CT in patients with advanced squamous cell carcinomas (SCCs) or ACCs. A recent study found that pembrolizumab was associated with a longer overall survival in advanced EC patients with PD-L1 CPS, while exhibiting a better safety profile and comparable quality of life. A second-line treatment option is now considered as the new standard of care for patients with EC and PD-L1 CPS [107].
A phase II study of pembrolizumab demonstrated significant improvements in overall survival over CT when used as the second-line treatment for advanced EC with PD-L1 CPS. In addition to pembrolizumab, pembrolizumab may also benefit PD-L1 CPS patients with EC. This drug is being studied in phase III KEYNOTE-590 (NCT03189719) for use as first-line treatment for advanced EC [107, 108].
Atezolizumab
It has been reported that health care providers have had poor success rates in treating patients with non-resectable locally advanced ESCCs using definitive CRT. It has been shown that the CR rate is strongly correlated with good prognosis, although the factors that determine the likelihood of CR have not been established. In patients with confirmed complete responses (cCR), PFS and OS rates were favorable. An important predictor of cCR was tumor length [109].
Chemoradiotherapy is used to treat ESCC when it is unresectable locally. It contains five fusidic acid metabolites and cisplatin. Only 11 percent to 25 percent of patients achieve a CR, and the median OS is nine to ten months for these patients. Due to their improved therapeutic efficacy, radiation and immunotherapy have received increasing attention.
The PFS and OS of patients with locally advanced NSCLC were significantly improved when platinum-based CRT was followed by anti-PD-L1 antibodies [40].
As part of the management of EC and GEJ adenocarcinoma throughout history, perioperative approaches have been used to improve the pathological CR rate (path CR), minimize or delay metastases, improve resectability, and enhance survival. An inhibitory receptor expressed on T cells, PD-1 and B7-1, performs by inhibiting the actions of PD-L1 on its receptors. As mentioned above, the humanized mAb atezolizumab targets PD-L1 by mimicking immunoglobulin G1. As a result of atezolizumab’s therapeutic binding to PD-L1, the T-cell response to tumors can be enhanced. Immunotherapy combined with CT may be of benefit to patients with localized EC or GEJ ACCs. When atezolizumab is administered with oxaliplatin and 5-fluorouracil, there is a high tumor regression rate when the drugs are combined. In order to support further research, atezolizumab should be administered to patients with EC and GEJ undergoing surgery in this trial [110].
Avelumab
There have been some reports of ICI being effective in treating a subset of patients and certain types of tumors. Avelumab mAb has been used in combination with CT to enhance antibody-dependent cytotoxicity. During the course of this study, avelumab was combined with chemotherapeutics, antiangiogenic drugs, and immunomodulators in order to enhance ICI regimens. This report will provide an overview of the current studies investigating the use of avelumab in combination with these agents. As demonstrated in clinical studies, avelumab is effective against Merkel cell carcinomas (MCCs), renal cell carcinomas (RCCs), and urothelial cancers when used alone in patients with cancer. RCC and urothelial cancer can be treated more successfully with avelumab and axitinib combined. In addition to these immunotherapy combination trials, several other immunochemotherapy trials failed as a result of factors disfavoring their use for ovarian cancer, GC, and NSCLC [111].
Esophageal cancer patients in stages II and III are generally treated with neoadjuvant chemoradiation followed by surgical resection. Approximately 50% of patients who have completed their initial treatment will experience recurrence of the disease. The outcomes are least favorable for resections with residual disease (75%), particularly for cases with ongoing lymph node involvement. Developing new strategies is necessary for improving outcomes. Numerous preclinical and clinical studies have demonstrated the synergy between radiation and immunotherapy. After exposure to CRT in EC, CD8+ T lymphocytes are detected infiltrating cancer cells. The microenvironment of the tumor induces an increase in the expression of PD-L1. Metastatic gastroesophageal cancer can be effectively treated with ICIs. The synergistic interaction between radiation and these agents may increase their effectiveness at earlier stages of the disease. Patients with resectable EC will participate in this trial to evaluate avelumab when combined with CT and radiation. Patients with resectable esophageal carcinoma will be studied in a phase I/II clinical trial using perioperative avelumab plus CRT [112].
Preoperative CT in combination with avelumab did not result in any unexpected side effects. It is likely that neoadjuvant chemoradiation coupled with immunotherapy will prove beneficial to patients with esophageal and GEJ tumors [113].
Nivolumab
Nivolumab inhibits the expression of PD-1 on activated T cells. Although nivolumab has a manageable safety profile, its activity has been encouraging [66].
There is a possibility that this drug could be useful for patients with advanced squamous-cell carcinoma who are unable to respond to existing treatments [79].
PD-1 can be treated with a mAb called nivolumab. It is the third phase of the ATTRACTION-3 project [62, 114].
Nivolumab was demonstrated to be superior to PD-L1-repeated antibodies in the KEYNOTE-181 phase III trial as second-line therapy for all patients with ESCC [107].
Pembrolizumab has been demonstrated to be a superior drug for the treatment of EC compared to other anticancer treatments in phase III trials [79].
Additionally, nivolumab has been developed for the treatment of lymphomas as well as cancers such as EC. ATTRACTION-1, a phase II study, evaluated nivolumab monotherapy for its efficacy. This drug is indicated for the treatment of patients with advanced EC, including EAC and esophageal squamous carcinoma, who have demonstrated a refractory or intolerant response to fluoropyrimidine-based CT, platinum-based CT, or taxane-based CT. This trial included 65 patients with ESCC. An analysis of a central database revealed that 18% of patients responding to nivolumab received the drug. Among other TRAEs, decreased appetite (3%), lung infection (3%), increased levels of creatinine phosphokinase (3%), and dehydration were the most commonly reported. It was demonstrated in the ATTRACTION-1 trial that monotherapy with nivolumab can successfully treat metastatic or recurrent ESCC [114].
When resectable EC is unresponsive to CT and surgery, adjuvant nivolumab monotherapy may be more beneficial. Neoadjuvant chemoradiation is often used in conjunction with neoadjuvant CT. The study of ICIs before and during surgery and CT is necessary to improve the outcomes of these procedures [115].
Camrelizumab
There is a better survival outcome when first-line CT combined with PD-1 inhibitors is used in advanced EC. Additionally, camrelizumab-CT resulted in the longest PFS. CT was the primary factor that improved ORR, in addition to nivolumab [116].
In a study combining PD-1 inhibitors with CT, patients’ OS, PFS, and ORR were improved at the expense of greater toxicity, which can be managed. The PFS benefit of sintilimab-chemo and camrelizumab-chemo was greater than that of CT, but there was no difference in OS benefit between checkpoint inhibitor strategies. As a combination of nivolumab and CT, nivolumab was found to improve ORR the most in patients with EC. A significant survival advantage was observed in the group with high PD-L1 expression [116].
Tislelizumab
Efficacy and safety of tislelizumab were evaluated in resectable ESCC patients receiving neoadjuvant treatment. There is evidence that tislelizumab plus CT as neoadjuvant therapy is effective in treating resectable ESCC, and the tolerability of the combination is acceptable. It is well known that tislelizumab in combination with CT results in high rates of MPR, pCR, and R0 resection [78, 117, 118].
A phase II study (NCT03469557) reported an overall response rate of 46.7% and a disease-free survival rate of 80% for GEJ ACCs, respectively [74].
The RATIONALE 302 phase III study produced similar results. Compared to CT, immune therapy with tislelizumab has already shown survival benefits in patients with advanced unresectable/metastatic ESCC [75].
Additionally, preliminary evidence of its antitumor activity has been demonstrated in various types of cancer [117, 119].
In light of strong data showing the safety and efficacy of combined CT and tislelizumab, it has been hypothesized that these treatments could provide an effective therapy for surgically resectable ESCC [78].
Durvalumab
Combining durvalumab monotherapy with durvalumab plus tremelimumab treatment had acceptable results in Asian patients, and the safety profiles were consistent with the published data. According to the preliminary results of the phase I study, durvalumab monotherapy and durvalumab plus tremelimumab had an acceptable safety profile and preliminary clinical activity. There may be potential benefit to further clinical development of durvalumab and tremelimumab in PD-1/PD-L1 and anti-CTLA-4 tumor types when combined with demonstrated safety profiles and preliminary efficacy. Asian patients with ESCC and head and neck squamous cell carcinoma (HNSCC) receiving durvalumab or tremelimumab did not show a significant difference in clinical outcomes between durvalumab monotherapy and durvalumab plus tremelimumab. The previous systemic CT had not been effective for these patients [120].
Ipilimumab
CTLA-4 and PD-1 ICIs are associated with a higher risk of adverse reactions. When patients have multiple adverse reactions to immunotherapy, higher doses of glucocorticoids must be administered and longer courses of therapy must be completed, which greatly increases the risk of complications, such as resurgence of the virus. According to the current guidelines, no information on adverse reactions or complications associated with multiple immune treatments is provided. Early diagnosis and treatment of immune-related adverse reactions are critical for patients with multiple adverse reactions. The future may see more efforts devoted to the study of immune-related complications and adverse reactions. For effective management of multiple immune-associated deleterious reactions, follow-up research is required [102, 121].
In addition to the effect of CTLA4 on interleukin-2 (IL-2) production, CTLA4 expression also plays a role in negatively regulating IL-2 production. In addition, CTLA-4 molecules on the surface of cancer cells prevent the immune system cells from entering the G1 phase, but also reduce the specific immune function and facilitate the escape of tumor cells from the immune system [84]. Several studies have shown that CTLA4 inhibitor can be effective in the treatment of cancer [85].
Currently, there are drugs targeting CTLA-4 in addition to ipilimumab and tremelimumab. The mAb ipilimumab, which blocks CTLA4, is an effective treatment for melanoma [28, 122].
There have been several medical studies conducted with ipilimumab for the treatment of EC, but no trials have taken place in humans. In the phase I/II CheckMate-032 trial (NCT01928394), ipilimumab and nivolumab were combined to treat solid tumors originating from the EC and tested for their efficacy and safety. Among gastroesophageal cancer patients receiving ipilimumab and nivolumab combined, Janjigian et al. reported a 6.9 months OS, and no adverse reactions were observed. This finding was consistent with previous reports [28, 123-127].
Cancers that are susceptible to ICIs include a variety of types. This type of therapy is effective only in a small number of patients, such as those who are resistant to PD-1, PD-L2, and programmed cell death-4 (PCD-4). Researchers have conducted preclinical studies showing promise for cancer treatment combinations that include PD-1 or PD-L1 inhibitors, cytotoxic CT, or CTLA-4 antibodies. The use of cytotoxic CT in combination with PD-1/PD-L1 inhibitors has been approved and is now used in clinical trials to treat NSCLC and small cell lung cancer that have shown positive results. Additionally, combination therapies using PD-1 (nivolumab) and CTLA-4 (ipilimumab) have shown survival benefits in patients with melanoma and renal cell carcinoma. There are several ongoing clinical trials evaluating ICI combination therapy for the treatment of other types of tumors. Although it is difficult to determine which patients will benefit from PD-1/PD-L1 inhibitor monotherapy, new complementary biomarkers should be developed. ICI combination therapy needs to be studied further to establish appropriate management strategies [128].
There has been speculation about the potential treatment targets CTLA-4 and PD-1 that may be used to combat cytotoxic CTLA-4. Many types of cancer have been treated with pembrolizumab, nivolumab, atezolizumab, and durvalumab, which are approved for PD-1 treatment, as well as PD-L1 inhibitors such as atezolizumab, durvalumab, ipilimumab and tremelimumab that target CTLA-4. In addition, many other clinical trials have reached an advanced stage of development. In addition to inhibiting acquired immune system tolerance, ICIs were observed to enhance antitumor T cell function in response to the tumor microenvironment and cancer cells overexpressing antitumor T cells. ICIs were ineffective as monotherapy for about half of the patients. Combining ipilimumab with nivolumab is commonly used to improve oncological outcomes. Despite promising results, ipilimumab and nivolumab have been hampered by safety concerns [129] (Table 1).
Table 1
Objectives and applications of monoclonal antibodies in esophageal cancer
Today, surgery, CT, and RT are the most common treatments for EC. There is currently a wide range of cancers that can be treated with ICIs, including EC. The combination of ipilimumab and nivolumab has been shown to benefit EC models. A combination therapy of nivolumab and ipilimumab showed promising results in patients with EC who did not respond to CT alone, as well as long-lasting antitumor effects [65, 129].
Conclusions
The current development of biomedical materials and therapeutic technologies may soon make it possible to treat EC more effectively [41]. Although checkpoint inhibitors may be effective in some patients, monotherapy may not be appropriate for all.
Generally, it has been found that combining these agents can improve outcomes in order to overcome this limitation. Whenever immune-associated deleterious effects are not detected and controlled in a timely manner, it is imperative to recognize and control these adverse effects [129]. A major part of immunotherapy is related to ICIs i.e., anti-PD-L1 and anti-CTLA-4, which play a critical role in treatment of patients with metastatic of EC.
It has been concluded that combination therapy could be more effective in patients with ESCC as compared to monotherapy. Actually, combination therapy (CT with immunotherapy) has been successful and reports of recurrence in patients with EC are rare.


