Purpose
Primary tracheal neoplasms are rare tumors. Their incidence is about 0.1 per 100,000 persons per year, representing approx. 0.2% of all respiratory tract tumors and 0.02% to 0.04% of all malignant tumors. About 90% of tracheal tumors in adults are malignant, compared with 10% to 30% of those in children [1]. Adenoid cystic carcinoma (ACC) is the second most common primary tracheal tumor after squamous cell carcinoma (SCC) [2, 3]. Rarity of this neoplasm results in an absence of prospective clinical trials investigating treatment effectiveness. Most of the authors prefer surgical resection, followed by post-operative radiation therapy in case of an incompleteness. There are no available prospective data on post-relapse treatment.
Case report
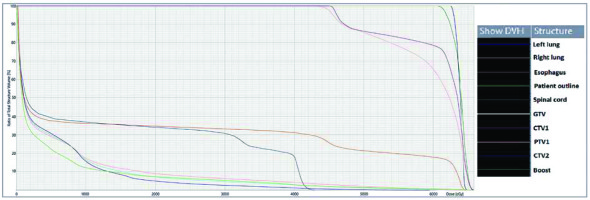
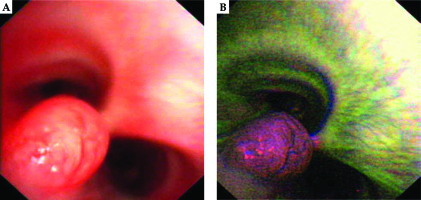
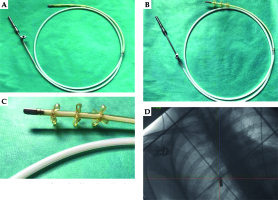
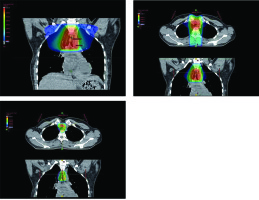
A 28-year-old woman diagnosed with tracheal ACC was presented to the Maria Sklodowska-Curie National Research Institute of Oncology in August 2012. The condition was diagnosed in 2008 when the patient was treated for dyspnea, which developed one month after undergoing a cesarean section. Diagnostic imaging revealed a mass measuring approximately 8-9 cm, located below vocal cords on the right posterolateral wall of the trachea. Rigid bronchoscopy and electrocautery excisional biopsy of a 2 cm fragment of the tumor were performed urgently under general anesthesia due to severe dyspnea, resulting in a diagnosis of ACC and improvement of general condition. Subsequently, the patient was qualified for surgical treatment, and a tracheal sleeve resection and anastomosis were performed, with a positive margin of resection (R1) found on a pathology report. The patient’s head was positioned anteriorly (chin-on-chest) after surgery. On the second day after surgery, she reported feeling weakness in her lower limbs. Magnetic resonance imaging (MRI) of the head and cervical spine showed no pathological findings. A neurological exam revealed moderate paresis, increased muscle tension, partial loss of superficial sensation in lower limbs, mild distal paresis with vivid tendon reflexes, and preserved superficial sensation in upper limbs. Analysis of cerebrospinal fluid (CSF) collected during lumbar puncture found no abnormalities. Physiotherapy improved the patient’s clinical condition and resolved associated symptoms. The patient was closely oncologically monitored after surgery, and a follow-up bronchoscopy in July 2012 revealed two polypoid lesions located in lower part of the trachea and right main bronchus (7 and 2 mm, respectively). A recurrence of ACC was confirmed via biopsies collected during bronchoscopy. Thoracic computed tomography (CT) found no evidence of other lesions. The patient was then referred to our department, where she was qualified for radiotherapy. A dose of 4,400 cGy/planning treatment volume (PTV) in fractions of 200 cGy/PTV was administered to the areas of recurrence (trachea, right main bronchus, and mediastinum), followed by a boost to a total dose of 6,400 cGy/PTV using standard fractionation. Radiotherapy planning was based on 6 MV and 15 MV photons using 3D conformal radiotherapy (3D-CRT) techniques. Lung volume receiving ≥ 20 Gy was 7.1% (V20, 7.1%) and ≥ 5 Gy was 29.6% (V5, 29.6%). Mean lung dose was 5.6 Gy and maximum spinal cord dose was 4.3 Gy. Volume of the esophagus, which received 60 Gy was 17.9%, and mean dose to the esophagus was 19.5 Gy (Figures 1 and 2). Two weeks after completion of external beam radiotherapy, endoluminal high-dose-rate (HDR) brachytherapy was performed. Bronchoscopy was carried out after local anesthetic administration and revealed the presence of residual tumor tissue located on the main carina in both white light and autofluorescence (Figure 3A, B). Fritz adjustable intralumenal applicator was used during the procedure (Figure 4A-C). Its design avoids administering a high mucosal dose by immobilizing and centering applicator within the lumen. The applicator was inserted into the trachea near the tumor using a modified Seldinger technique and fixed in the right main bronchus. Orthogonal localization film was taken to ensure correct positioning of the applicator and to set a 6 cm target length (Figure 4D), which included residual tumor and 2 cm margins both proximally and distally. Two 5 Gy fractions were applied at one-week intervals prescribed at reference points one centimeter from the source. According to the Radiation Therapy Oncology Group (RTOG) acute radiation morbidity scoring criteria, the patient developed radiation-induced grade 2 esophagitis and was treated with analgesics and a pureed diet, resulting in a significant improvement. Radiotherapy was completed in October 2012, and since then, the patient remains in observation. Follow-up bronchoscopy and CT scans found no signs of a recurrence, with the most recent scan performed in October 2020. The patient stays in a very good overall condition, with ECOG = 0.
Fig. 1
Radiotherapy plan presented in computed tomography scans of the treated region with 3D reconstruction. Isodoses levels are presented in the dose color wash method from dose 1,300 cGy (blue) to 6,580 cGy (red)
Discussion
Adenoid cystic carcinoma arises from a minor salivary and serous glands presence within tracheal submucosa, and is morphologically similar to primary tumors of salivary glands [4]. ACC usually demonstrates exophytic growth leading to tracheal lumen narrowing [2], and is characterized by submucosal and perineural spread. Regional lymph node involvement or distant metastases are detected at the time of diagnosis in up to 10% of patients [2]. Some ACC are associated with slower rates of disease progression, with a five-year survival rate of about 90% and less than 40% survival at 15 years. More aggressive cases are associated with advanced-stage neoplasia at the time of diagnosis and more frequent incidence of recurrence and distant metastases. Distant or local recurrence can occur after a variable period of time [2]. ACC develops predominantly in the fourth and fifth decade of life, with similar frequency in men and women [2]. The etiology is unknown, but is not associated with smoking.
Radical surgical management is the treatment of choice whenever possible. The extent of resection depends on many factors, such as tumor size, location, and local extent [2]. The process of patient qualification for surgery should also include additional factors, including the amount of normal trachea that would remain after planned resection, any history of previous mediastinal surgeries or radiotherapy, age, and patient’s physique [5]. Older patients with a short, stiff neck, are more likely to receive limited surgical resection in contrast to younger, lean, and tall patients. Importantly, this kind of surgery often requires particular patient’s positioning, which must be modified throughout the procedure. Neck extension is also limited during post-operative care [5, 6]. The most common complications of tracheal surgery include anastomotic leak (0.04-23.8%), chyle leak (4.3-22%), new recurrent laryngeal nerve paralysis (0.04-55%), esophageal fistula (5-6%), unplanned permanent tracheostomy (4.3-50%), respiratory failure or prolonged intubation (10%), tracheal stenosis (5%), and wound infection (1.4-11%) [7].
Until now, a rare event of cervical spinal cord disfunction was described in only some case reports, with one related to ACC [8-10]. Excessive neck flexion after surgery and sitting position resulting in low blood pressure appear to be the main causal factors. Reviewing the available literature, Windfuhr et al. presented possible etiologies, which must be considered when spinal cord disfunction is diagnosed [11]. According to the authors, an informed consent for surgery should include the possibility of such a rare complication, due to its unpredictability. Appropriate post-operative care consisting of daily neurological exams and avoidance of excessive neck flexion after surgery was also highlighted. In the presented case, the patient’s head was positioned anteriorly during the post-operative period. Unfortunately, the detailed data regarding post-operative care, diagnostic processes, and treatment were limited due to main surgery having been performed in a district hospital. However, the consistency of clinical outcome and evidence coming from case reports allow us to suspect a diagnosis of cervical spinal cord disfunction.
The role of post-operative radiotherapy in ACC is unclear. Majority of publications recommend radiotherapy in cases of incomplete resection; however, this suggestion was not based on results of randomized prospective clinical trials [12-14]. Adjuvant radiation in a variety of dosages was used by Maziak et al. in 25 of 32 patients managed by primary resection. There was no statistically significant difference in survival between these 25 patients receiving adjuvant radiation and the seven patients who did not. Six of the seven patients, who had no adjuvant radiotherapy, accomplished a complete resection. The authors compared their results with observations reported in previous publications. They concluded that it appears logical to assume that adjuvant radiotherapy may be beneficial and likely to delay or even reduce the incidence of local recurrence within the airway, even though the role of adjuvant radiotherapy was impossible to evaluate with certainty [15]. The standard dose for adjuvant treatment is 60 Gy in fractions of 2 Gy, given 5 times per week over 6 weeks [2]. In case of gross residual tumor mass, the dose should be increased to 68-70 Gy in fractions of 2 Gy [2]. Post-operative radiotherapy planning must be based on pre-surgical CT scans [16].
Radical radiotherapy alone is performed in patients who are not candidates for radical resection surgery due to medical contraindications or lack of consent. Adequately staged patients in good general condition are eligible for this kind of treatment. ACC exhibits low radiation sensitivity, and the required dose of irradiation to maintain local cancer control was estimated as 70 Gy (35 fractions over 7 weeks) [2, 16]. Radiotherapy should be planned using conformal techniques, preferably intensity-modulated radiation therapy (IMRT) [16]. Endotracheal brachytherapy with 8-15 Gy may improve local control when applied after external-beam radiotherapy (60-68 Gy) [2]. Further research should be conducted to determine the maximum and optimum dose of endotracheal brachytherapy as a method, which can potentially increase the total dose when added to external beam radiotherapy [2].
Radiotherapy in tracheal cancer patients is well-tolerated in terms of toxicity. In a retrospective study including 48 radiotherapy patients, most of them (31 patients) did not report any side effects. In the rest of analyzed group, dysphagia was the most frequently reported adverse event (8 patients), followed by dyspnea (3 patients), oedema (2 patients), and pain (2 patients). One person suffered from deterioration of their general condition. In most cases, toxicity was evaluated as low-grade. Amongst 8 patients receiving brachytherapy, hemorrhage was reported only once. The rest of patients did not develop any clinical signs of toxicity after this treatment [17].
Lack of adjuvant radiotherapy due to post-operative neurological complications was a probable reason for ACC recurrence in our patient. Administration of external beam radiotherapy, followed by brachytherapy boost after incomplete resection of the recurrent disease, may lead to long-term locoregional control.